Construction of isovalerylspiramycin I gene engineering strain
A technology of isovalerylspiramycin and genetically engineered strains, which is applied in the fields of genetic engineering, plant genetic improvement, biochemical equipment and methods, etc., to achieve the effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
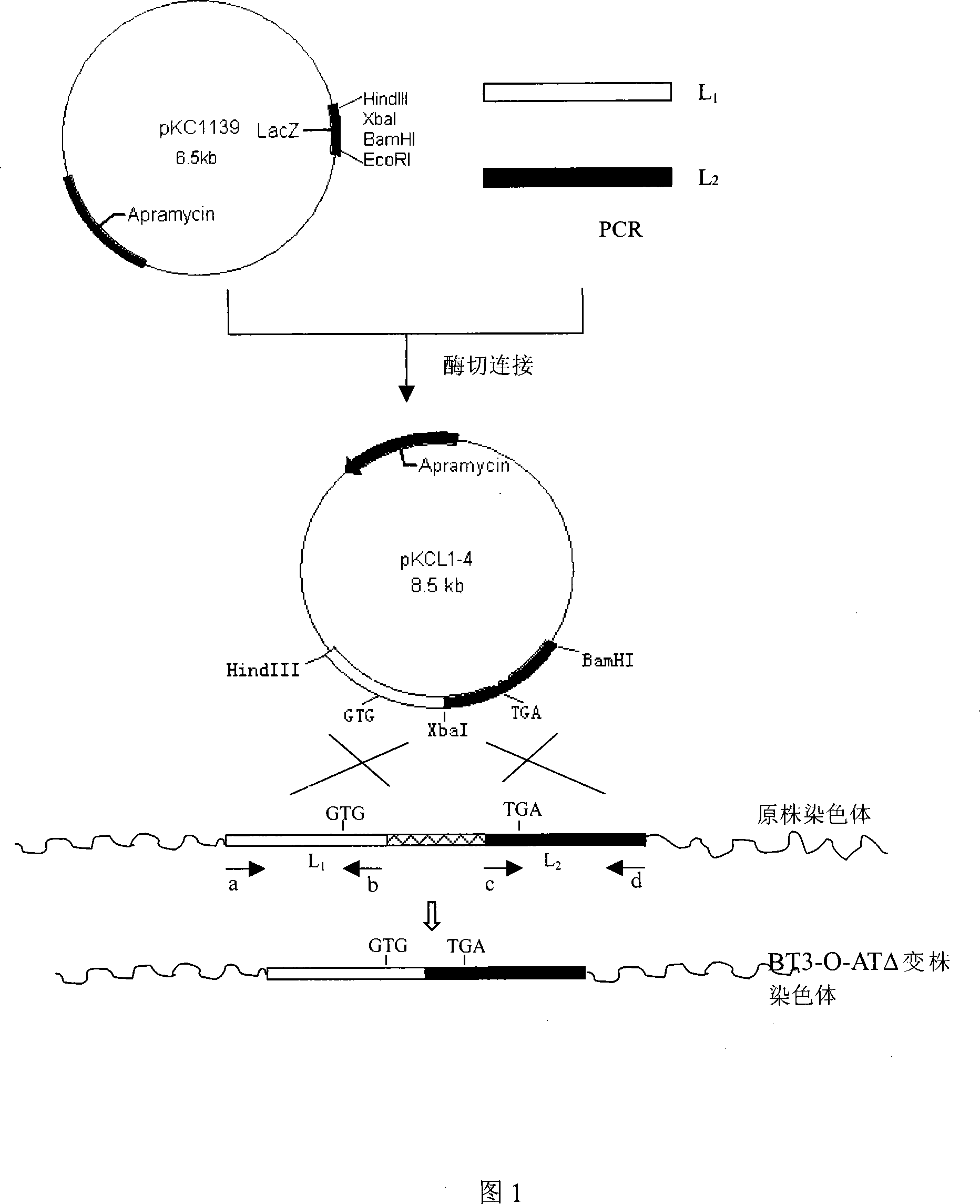
[0033] Construction of recombinant vector pKCL1-4
[0034] According to the 3-O-acyltransferase gene and its flanking sequence [NCBIDQ642742] obtained by our laboratory, two pairs of primers were designed and inserted into the corresponding restriction sites (the relative positions of the primers on the chromosome are shown in Figure 1):
[0035] L1 upstream primer (a) GCG AAGCTT TGCCCTGGCAATTGCAGTGTCAG HindIII
[0036] Downstream primer (b) GCG TCTAGA GCCGAACGTGACGATGTGCAGCG XbaI
[0037] L2 Upstream Primer (c)GTC TCTAGA CAGTGGTCCTTCGCCTTCTATCT XbaI
[0038] Downstream primer (d)GAC GGATCC TCGTCGCGCAGCAGGTCGTTGAG BamHI
[0039] Conventional PCR amplification was performed to obtain a fragment homologous to two parts of the 3-O-acyltransferase gene (left arm L 1 , 1.0kb; right arm L 2 , 1.0kb). Both ends of L1 carry HindIII and XbaI restriction sites, and both ends of L2 carry XbaI and BamHI sites. Will L 1 , L 2 The fragment was ligated with the thermosens...
Embodiment 2
[0040] Construction of the genetically engineered strain of Bitespiramycin 3-O-acyltransferase gene disruption
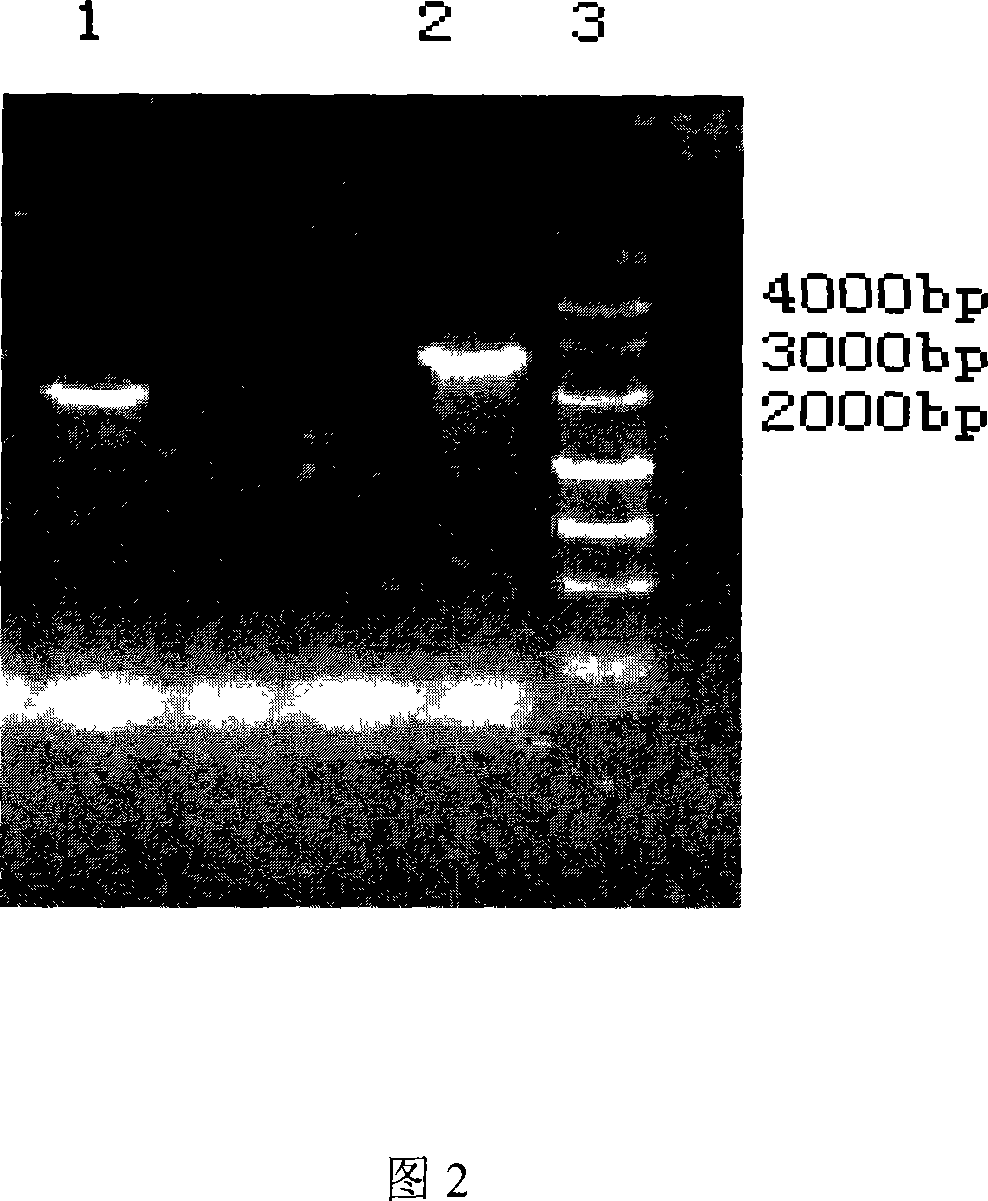
[0041] Bitespiramycin-producing bacteria were cultured at 28°C for 7-10 days on slant medium [Wang Yiguang et al., Acta Biological Engineering, 1992 8(1): 1-14], according to the literature [D.A.Hopwood et al. Geneticmanipulation of Streptomyces, A The method described in Laboratory Manual, Norwich; JohnInnesFoundation UK, 1985] prepared protoplasts, and pKCL1-4 was introduced into the Bitespiramycin producing bacteria through the protoplast transformation method, and Am resistance was used as a selectable marker to obtain transformants; The non-resistant plate was cultured and subcultured at 37°C, and a single colony was isolated to obtain an Am-sensitive strain (called BT3-O-ATΔ strain).
[0042] The genomic DNA of the mutant strain and the original strain were extracted as templates, and primers a and d were paired for PCR amplification and electrophoresis ident...
Embodiment 3
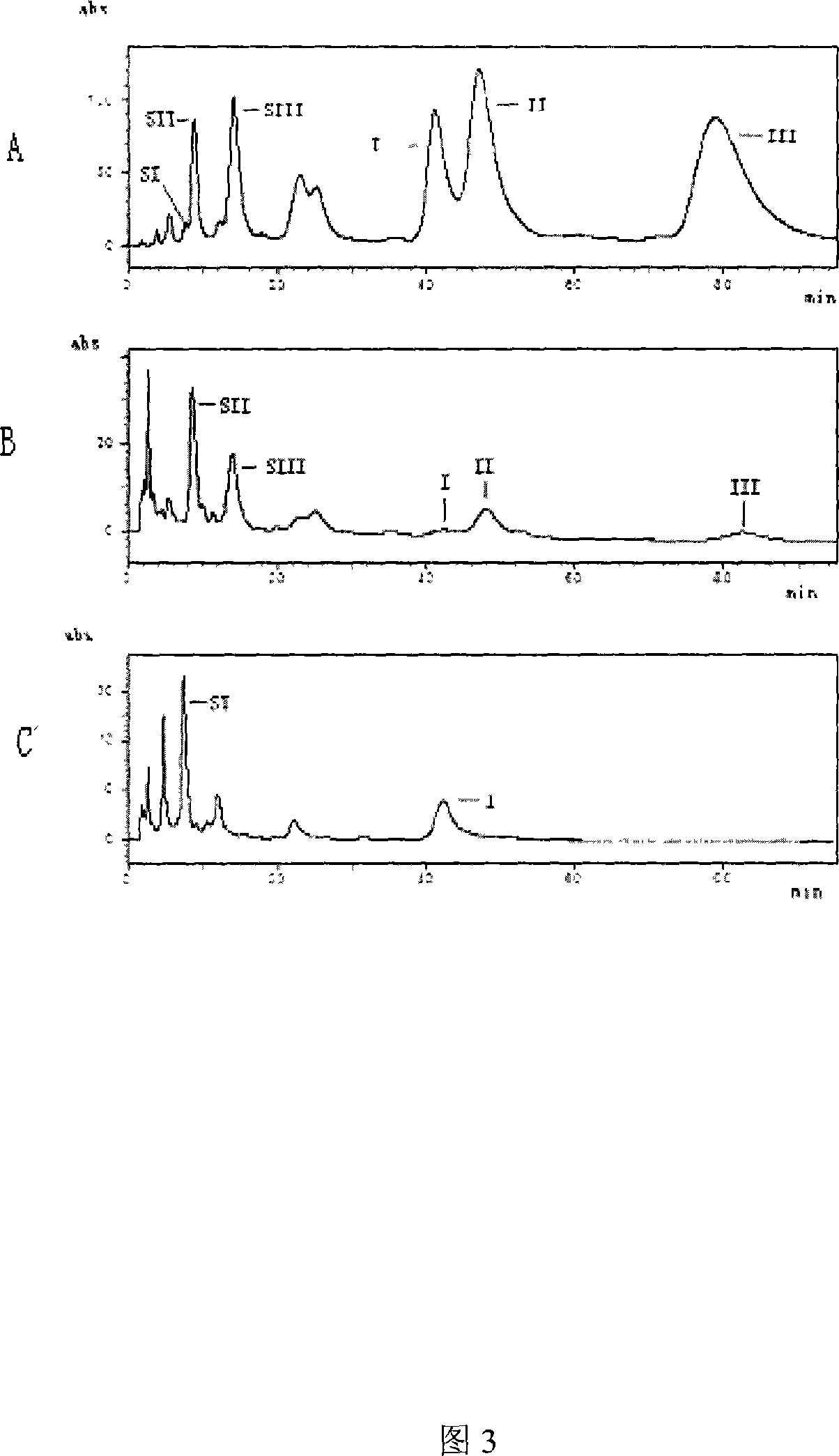
[0043] HPLC detection of the fermentation product of the genetically engineered strain of Bitespiramycin 3-O-acyltransferase gene disruption
[0044] The fermentation and preliminary extraction of the BT3-O-ATΔ mutant strain were carried out according to the literature [Wang Yiguang et al., Acta Bioengineering, 1992, 8(1): 1-14]. After the initial extract of the fermentation broth was evaporated to dryness, it was dissolved in a small amount of methanol, and the Shimadzu LC-10ATvp liquid chromatograph was used, with a second tube array detector, and the chromatographic column was Kromasil C184. Sodium hydrogen solution (53 / 47), the flow rate is 1mL / min. With bitspiramycin pure product as contrast, take the retention time of bitspiramycin main component as standard [Chinese Journal of Antibiotics such as Jiang Wei. 2002,27 (7): 387-391], get 5ul fermented liquid, The initially extracted samples were tested by HPLC. The HPLC quantitative analysis results of the proportion of m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com