Method for preparing super-fine vanadic-acid bismuth-yellow pigment
A technology of fine bismuth vanadate and yellow pigments, applied in the chemical field, can solve the problems of single raw material, complex manufacturing process, unsatisfactory, etc., achieve mild reaction conditions and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
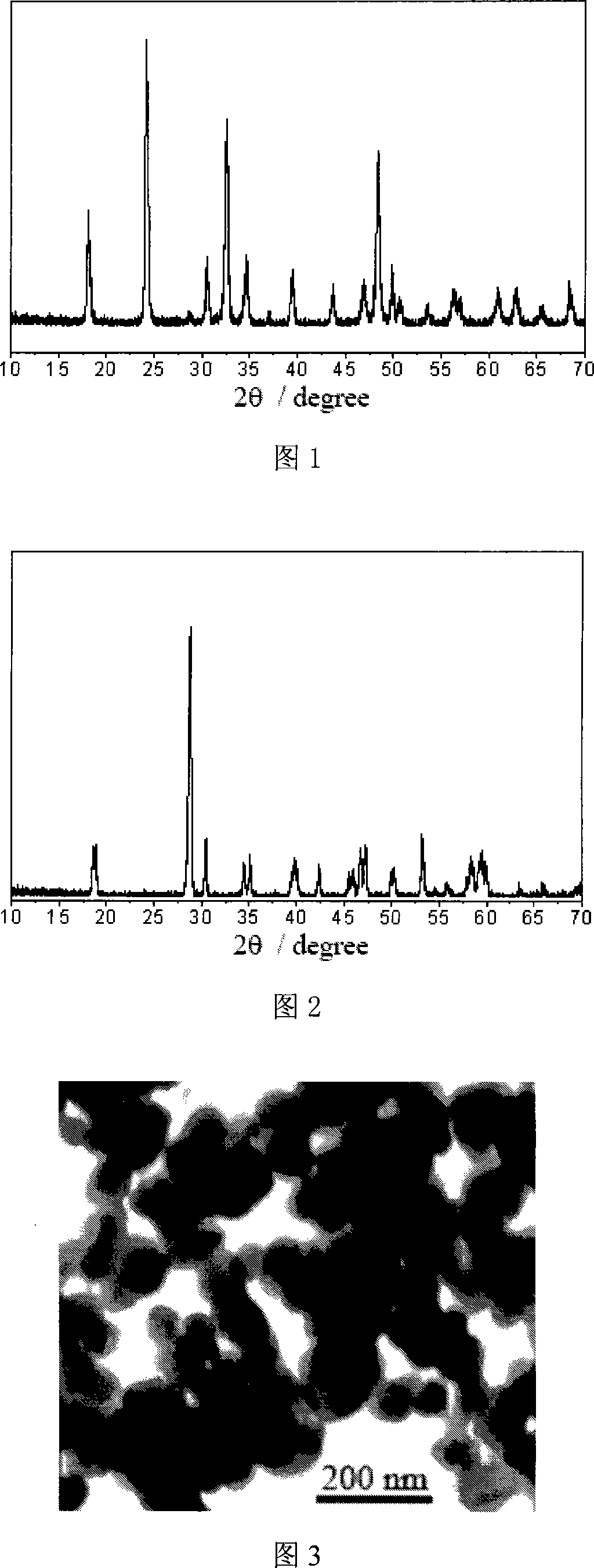
[0031] Dissolve bismuth nitrate and ammonium metavanadate in 4 mol L -1 In the nitric acid aqueous solution, the concentration of bismuth nitrate and ammonium metavanadate is 0.1mol L -1 , then add the nitric acid aqueous solution of ammonium metavanadate to the nitric acid aqueous solution of bismuth nitrate at room temperature, and control the V: Bi molar ratio to be 1: 1, and adjust the pH value to 2 by dropping ammonia solution diluted 1 times with distilled water , stirred and reacted for 2 hours, the precipitate was filtered, washed and dried to obtain light yellow powder. X-ray diffraction test shows that the product is tetragonal zirconium silicate structure. Accompanying drawing 1 is the X-ray diffraction pattern of product. The light yellow powder was heat-treated at 500° C. for 2 hours to obtain a dark yellow powder. X-ray diffraction test shows that the product is monoclinic scheelite structure. Accompanying drawing 2 is the X-ray diffraction pattern of the pro...
Embodiment 2
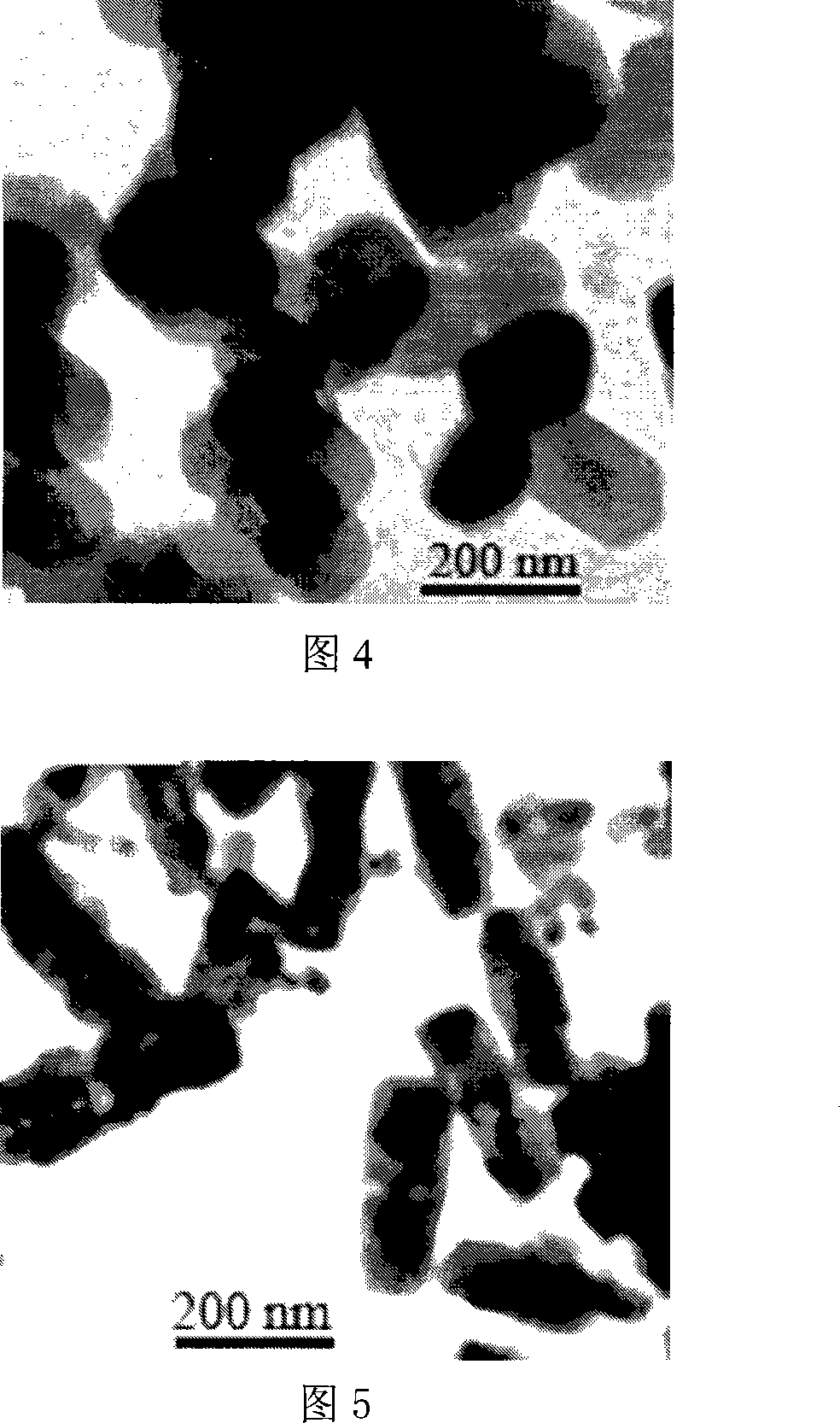
[0033] Dissolve bismuth nitrate and sodium metavanadate in 4 mol L -1 In the nitric acid aqueous solution, the concentration of bismuth nitrate and sodium metavanadate is 0.15mol L -1 , then add the nitric acid aqueous solution of sodium metavanadate to the nitric acid aqueous solution of bismuth nitrate at room temperature, and control V: Bi molar ratio is 1: 1, by dropwise adding 2mol L -1 Aqueous sodium hydroxide solution was used to adjust the pH value to 2, and the reaction was stirred for 1 hour. The precipitate was filtered, washed, and dried to obtain a yellow powder. X-ray diffraction test shows that the product is tetragonal scheelite structure. Accompanying drawing 4 is the transmission electron micrograph of product, proves that product is flaky bismuth vanadate.
Embodiment 3
[0035] Dissolve bismuth nitrate and ammonium metavanadate in 5 mol L -1 In the nitric acid aqueous solution, the concentration of bismuth nitrate and ammonium metavanadate is 0.2mol L -1 , and then add the nitric acid aqueous solution of ammonium metavanadate to the nitric acid aqueous solution of bismuth nitrate at 50 ° C, and control the V: Bi molar ratio to be 1: 1, by dropping 2mol L -1 Potassium hydroxide aqueous solution to adjust the pH value to 1, stirred and reacted for 0.5 hours, filtered the precipitate, washed, and dried to obtain a yellow powder with a green phase. X-ray diffraction test shows that the product is monoclinic scheelite structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com