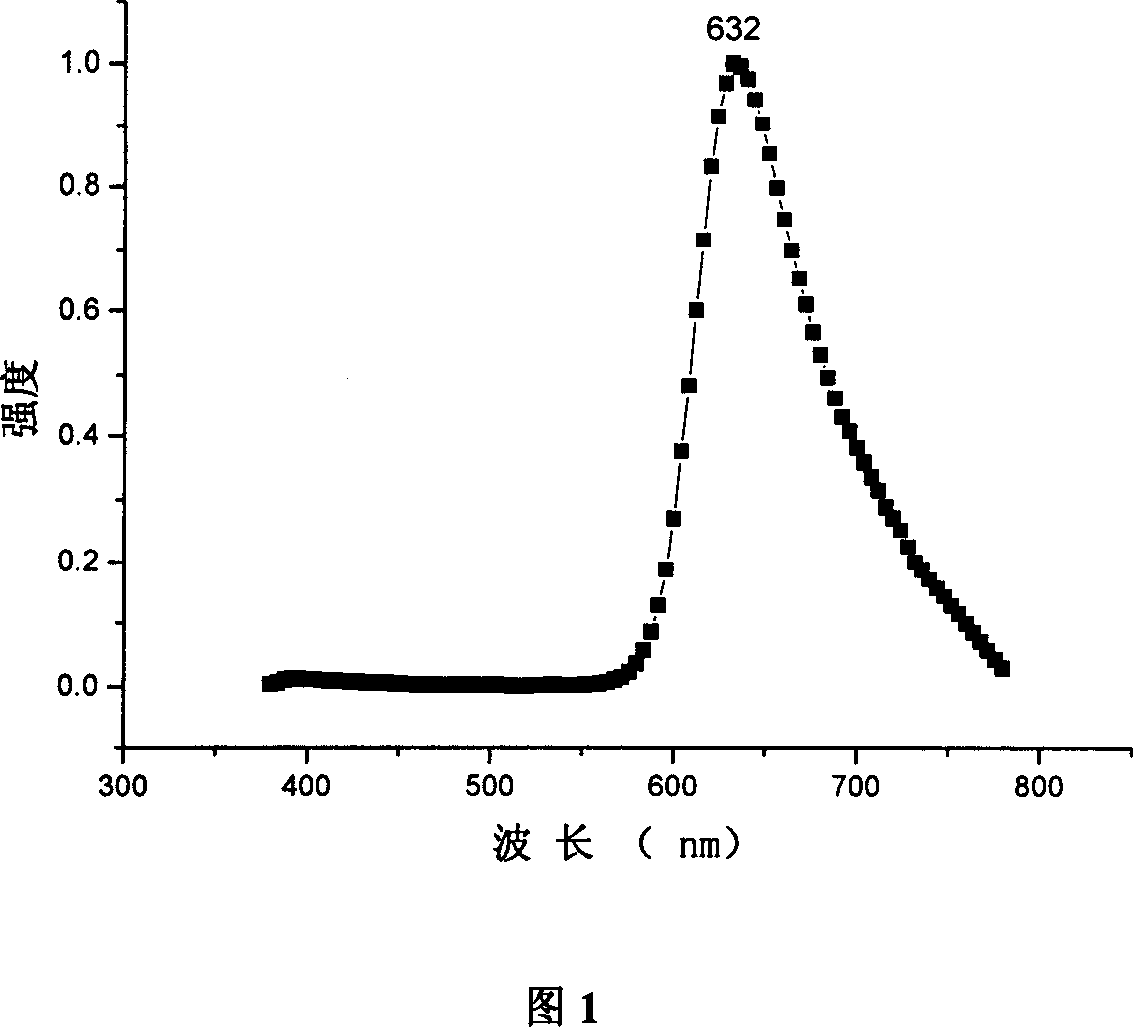
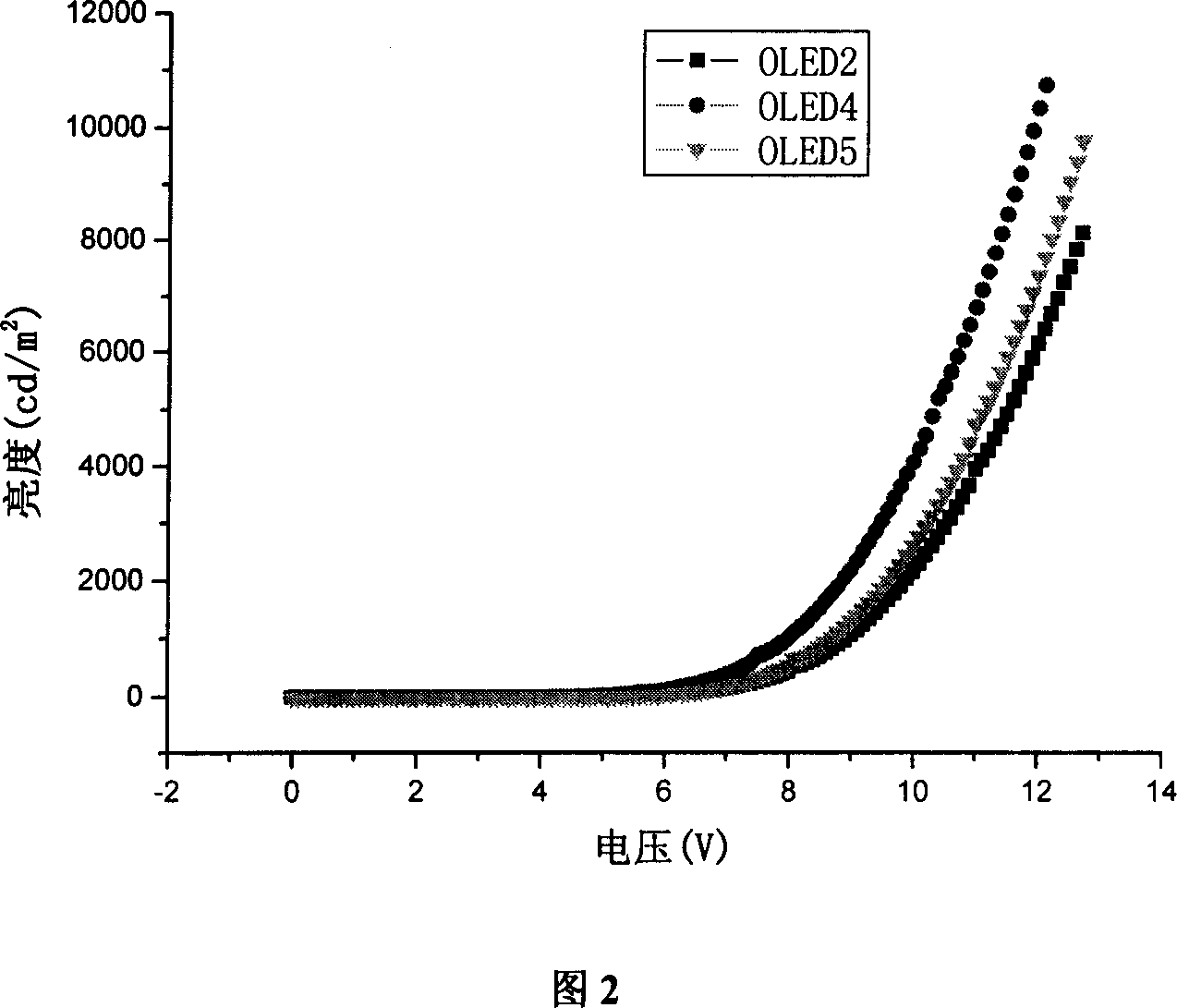
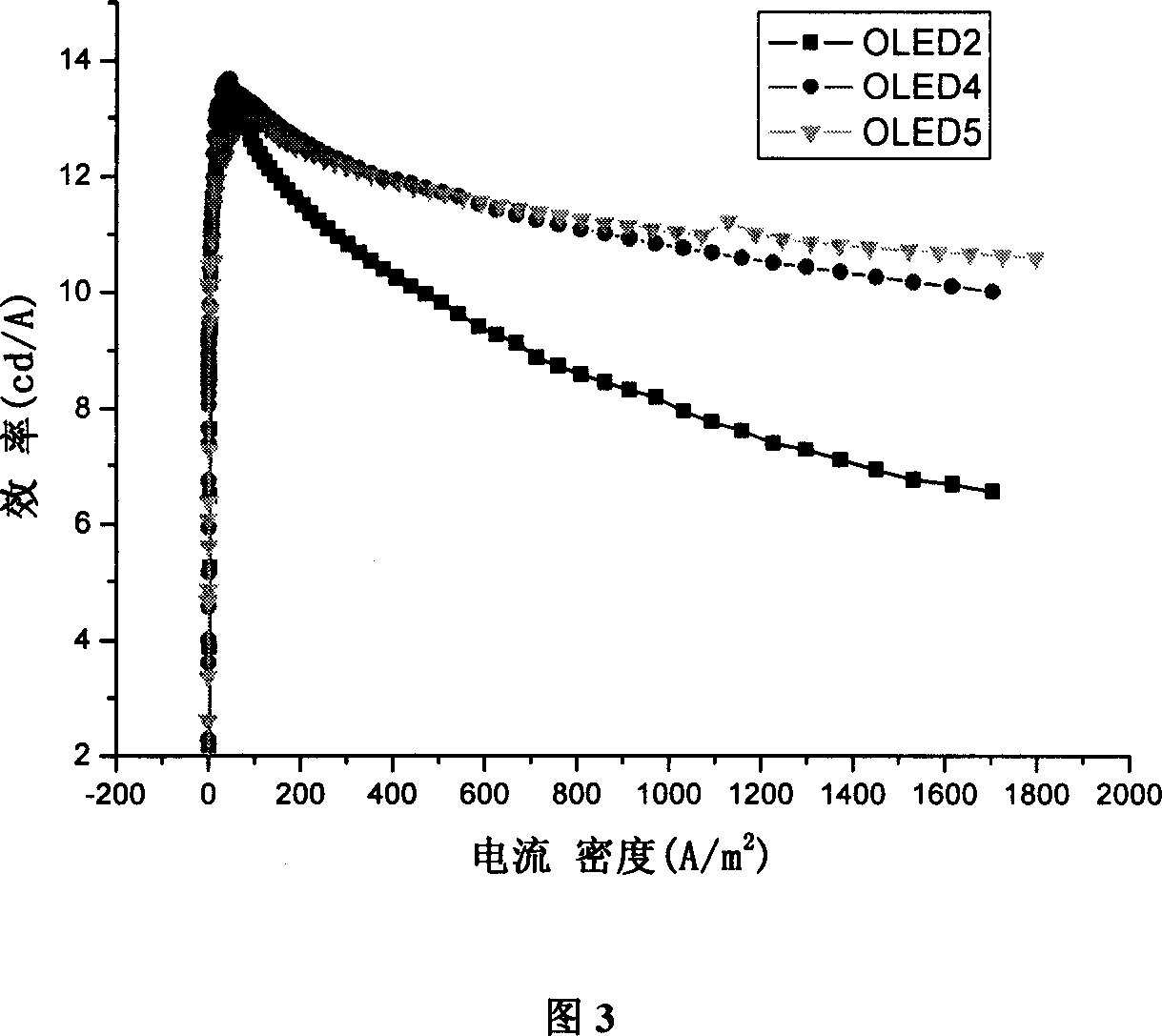
Organic electroluminescent phosphorescence luminescent material and application thereof
A luminescent material, electrophosphorescence technology, applied in the fields of organic electroluminescence materials, red phosphorescence materials, and organic electroluminescence devices to achieve high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0035] Preferred embodiment: The ligands in the compounds of the present invention are all prepared by condensation of o-aminonaphthalenone (or aldehyde) and α-methyl (or methylene) ketone.
[0036] Ligand preparation:
[0037] 1. The ligands from i-1 to i-12 are prepared according to the following method.
[0038] (a) Preparation of ligands from i-1 to 1-4:
[0039] Reaction formula:
[0040]
[0041] Process: The raw material 2-amino-1-naphthaldehyde was prepared according to literature (Emmanuelle Tarfarel, et al: Journal of Organic Chemistry, 1994, 59, 823-828).
[0042]In a 100mL three-neck flask equipped with a magnetic stirring and reflux condenser, add 1.5mmol 2-amino-1-naphthaldehyde, 1.4mmol acetophenone, 0.7mL saturated KOH ethanol solution and 30mL ethanol, and reflux for 15 hours under the protection of argon . After natural cooling, add 30mL of water, extract twice with dichloromethane, and use Na 2 SO 4 Drying, rotary evaporation to remove the solvent, ...
Embodiment 1
[0064] Example 1: Compound i-1
[0065] Reaction formula:
[0066]
[0067] process:
[0068] In a 100mL three-necked flask equipped with mechanical stirring, reflux condensing device and nitrogen protection device, add in sequence: the ligand of i-1 (15mmol, 3.8g), iridium trichloride hydrate (6mmol, 2.01g), ethylene glycol Monoethyl ether 45mL, distilled water 15mL. Evacuate, fill with N 2 , repeated 5 times to remove the oxygen in the system. Heat at 110°C and reflux for 24 hours. After natural cooling, add 10 mL of distilled water, shake, filter with suction, wash with water, and wash with ethanol. After drying in vacuo, 3.5 g of a crude dichloro-bridged intermediate was obtained as a dark red solid with a yield of 79.5%. Column separation and purification.
[0069] In a 50ml three-necked flask equipped with magnetic stirring and reflux condenser, the above intermediate (1mmol, 1.47g), acetylacetone (2.5mmol, 0.25g, 0.26mL), anhydrous Na 2 CO 3 (2.2mmol, 0.233g...
Embodiment 2
[0071] Embodiment two: compound i-2
[0072] Reaction formula:
[0073]
[0074] Process is the same as embodiment one, just changes the acetylacetone of the second step into picolinic acid.
[0075] Product MS (m / e): 823; Elemental Analysis (C 44 h 28 IrN 3 o 2 ): theoretical value C: 64.22%, H: 3.43%, N: 5.11%; measured value C: 64.85%, H: 3.20%, N: 5.04%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com