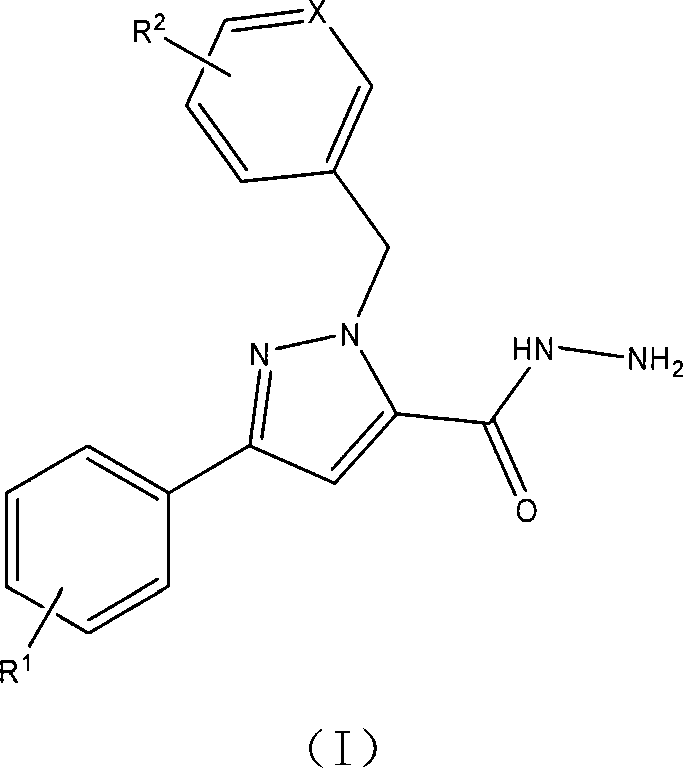
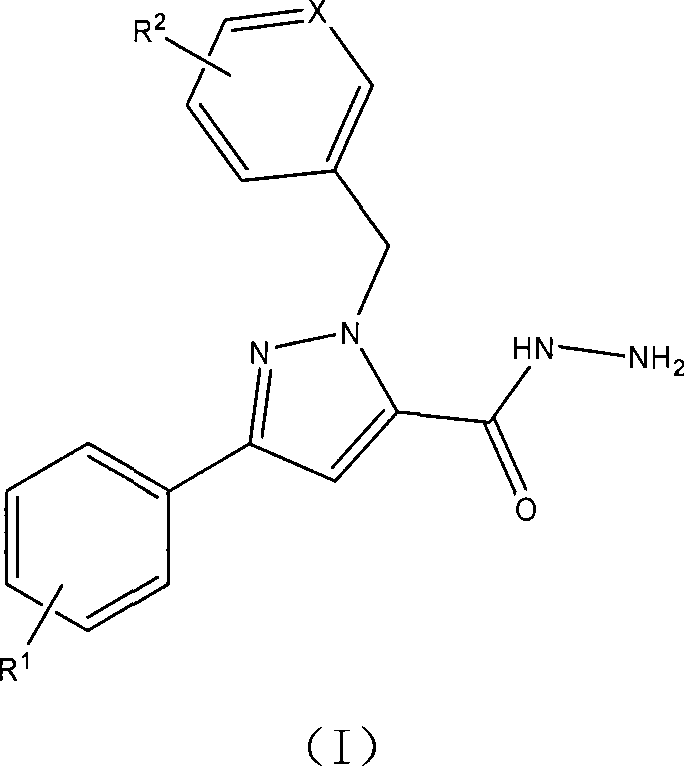
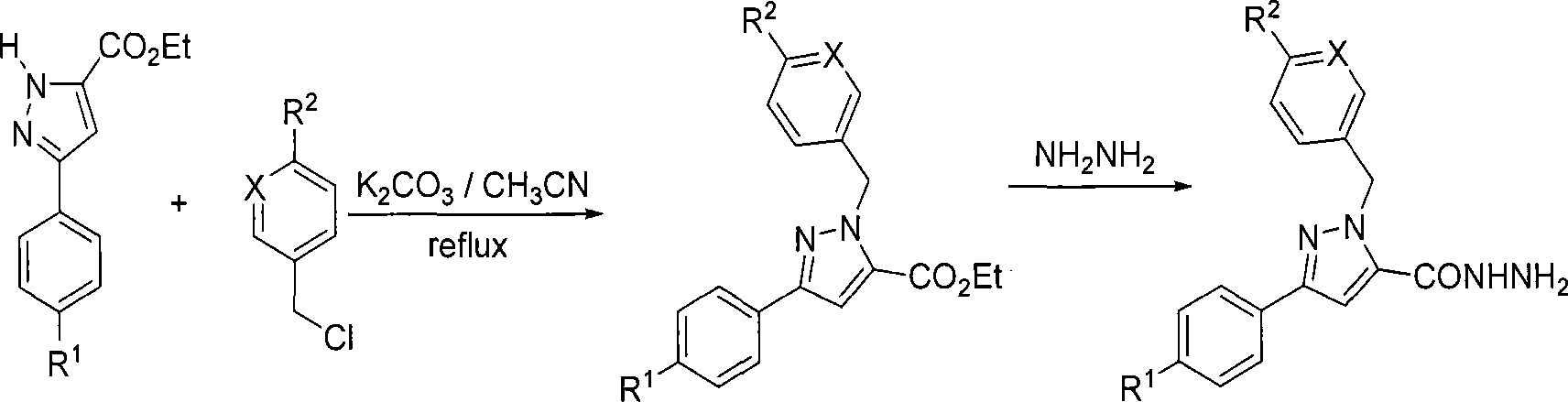
1-arylmethyl-3-aryl-1h-pyrazole-5-carbohydrazide derivative, preparation method and application thereof
A methyl and pyrazole technology, applied in the field of pyrazole carbohydrazide derivatives and their preparation, can solve unseen problems and achieve the effect of great application and development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Preparation of 1-benzyl-3-phenyl-1H-pyrazole-5-carbohydrazide
[0046] 1) In a 100 mL round bottom flask, add 0.690 g potassium carbonate (0.005 mol), 1.080 g 3-phenyl-1H-pyrazole-5-carboxylic acid ethyl ester (0.005 mol), 0.633 g benzyl chloride (0.005 mol) ) and acetonitrile (25 ml), a reflux condenser was installed, and a drying tube was connected to the upper part. Heating and refluxing for 8 hours, the reaction was carried out until the raw material was completely consumed, and the reaction end point was detected by TLC. Concentrate under reduced pressure, remove the solvent, add ethyl acetate (30 mL) to dissolve the product, filter, concentrate the filtrate, and use ethyl acetate-petroleum ether (V / V=1 / 2) as the eluent to separate the residue by silica gel column chromatography ( 100-200 mesh silica gel) to obtain 1-benzyl-3-phenyl-1H-pyrazole-5-carboxylic acid ethyl ester with a yield of 79%.
[0047] 2) Add 1.2 ml of 80% hydrazine hydrate to 0.336 g...
Embodiment 2
[0060] Example 2: Preparation of 1-(4-tert-butylbenzyl)-3-phenyl-1H-pyrazole-5-carbohydrazide
[0061] 1) In a 100 mL round bottom flask, add 0.690 g potassium carbonate (0.005 mol), 1.080 g 3-phenyl-1H-pyrazole-5-carboxylic acid ethyl ester (0.005 mol), 0.913 g p-tert-butylbenzyl Chlorine (0.005 mol) and acetonitrile (25 mL) were installed in a reflux condenser with a drying tube at the top. It was heated to reflux for 6 hours, and the reaction was carried out until the starting material was completely consumed, and the reaction end was detected by TLC. Concentrate under reduced pressure, remove the solvent, add ethyl acetate (30 mL) to dissolve the product, filter, concentrate the filtrate, and use ethyl acetate-petroleum ether (V / V=1 / 2) as the eluent to separate the residue by silica gel column chromatography ( 100-200 mesh silica gel) to obtain 1-(4-tert-butylbenzyl)-3-phenyl-1H-pyrazole-5-carboxylic acid ethyl ester with a yield of 80%.
[0062] 2) To a solution of 0.36...
Embodiment 3
[0075] Example 3: Preparation of 1-(3-(6-chloropyridine)methyl)-3-phenyl-1H-pyrazole-5-carbohydrazide
[0076] 1) In a 100 ml round bottom flask, add 0.690 g potassium carbonate (0.005 mol), 1.080 g 3-phenyl-1H-pyrazole-5-carboxylic acid ethyl ester (0.005 mol), 0.810 g 2-chloro-5- Chloromethylpyridine (0.005 mol) and acetonitrile (25 mL) were installed in a reflux condenser, and the upper part was connected to a drying tube. The mixture was heated to reflux for 4 hours, and the reaction was carried out until the starting material was completely consumed, and the end of the reaction was detected by TLC. Concentrate under reduced pressure, remove the solvent, add ethyl acetate (30 mL) to dissolve the product, filter, concentrate the filtrate, and use ethyl acetate-petroleum ether (V / V=1 / 2) as the eluent to separate the residue by silica gel column chromatography ( 100-200 mesh silica gel) to obtain 1-(3-(6-chloropyridine)methyl)-3-phenyl-1H-pyrazole-5-carboxylic acid ethyl est...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com