Polyamide bisbenzimidazole compound, preparation method and application thereof
A technology of bisbenzimidazole and compound, applied in the field of compound and preparation thereof, can solve the problems such as difficult utilization of fragments and no practical value, and achieve the effects of saving reaction cost and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
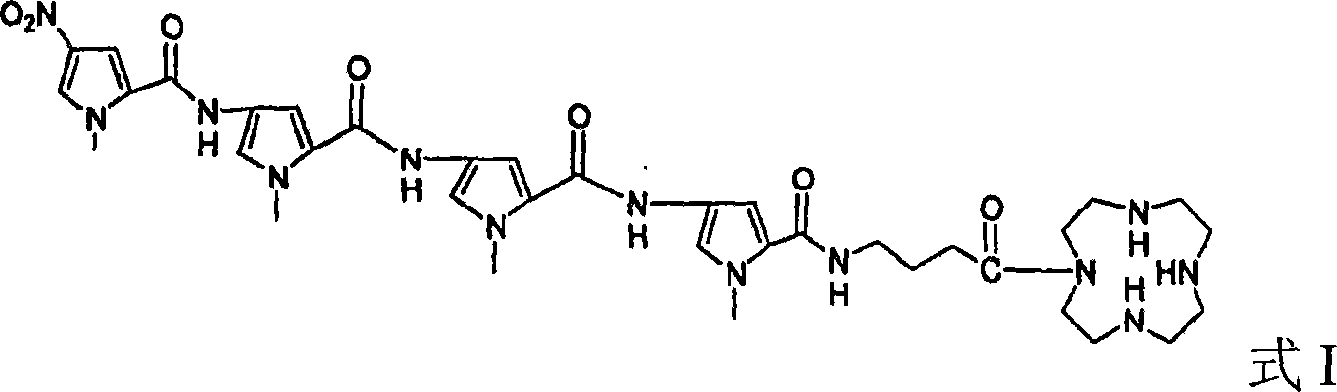
[0024] Example 1: N,N'-(benzimidazolyl)-λ-(4-(4-(4-(N-methylpyrrole-2-acyl)amino-N-methylpyrrole-2- Preparation of acyl)amino-N-methylpyrrole-2-acyl)amino-N-methylpyrrole-2-acyl)aminobutanamide
[0025] (1) 4-(4-(4-(4-nitro-N-methylpyrrole-2-acyl)amino-N-methylpyrrole-2-acyl)amino-N-methylpyrrole-2-acyl ) Preparation of amino-N-methylpyrrole-2-acyl)aminobutyric acid
[0026] ① 1mol of N-methylpyrrole and 1mol of trichloroacetyl chloride are acylated at room temperature to obtain N-methyl-2-trichloroacetylpyrrole;
[0027] ②1mmol N-methyl-2-trichloroacetylpyrrole and 1mmol HNO 3 And 0.09mmol of H 2 SO 4 Carry out nitration reaction at -10°C to obtain 4-nitro-2-trichloroacetyl-N-methylpyrrole;
[0028] ③1mol 4-nitro-2-trichloroacetyl-N-methylpyrrole and 1mol NH 2 CH 2 CH 2 CH 2 CO 2 Et reacts at room temperature to obtain ethyl λ-(4-nitro-N-methylpyrrole-2-acyl)aminobutyrate;
[0029] ④1mol of λ-(4-nitro-N-methylpyrrole-2-acyl)aminobutyric acid ethyl ester is catalyzed by 0.1mol 10%...
Embodiment 2
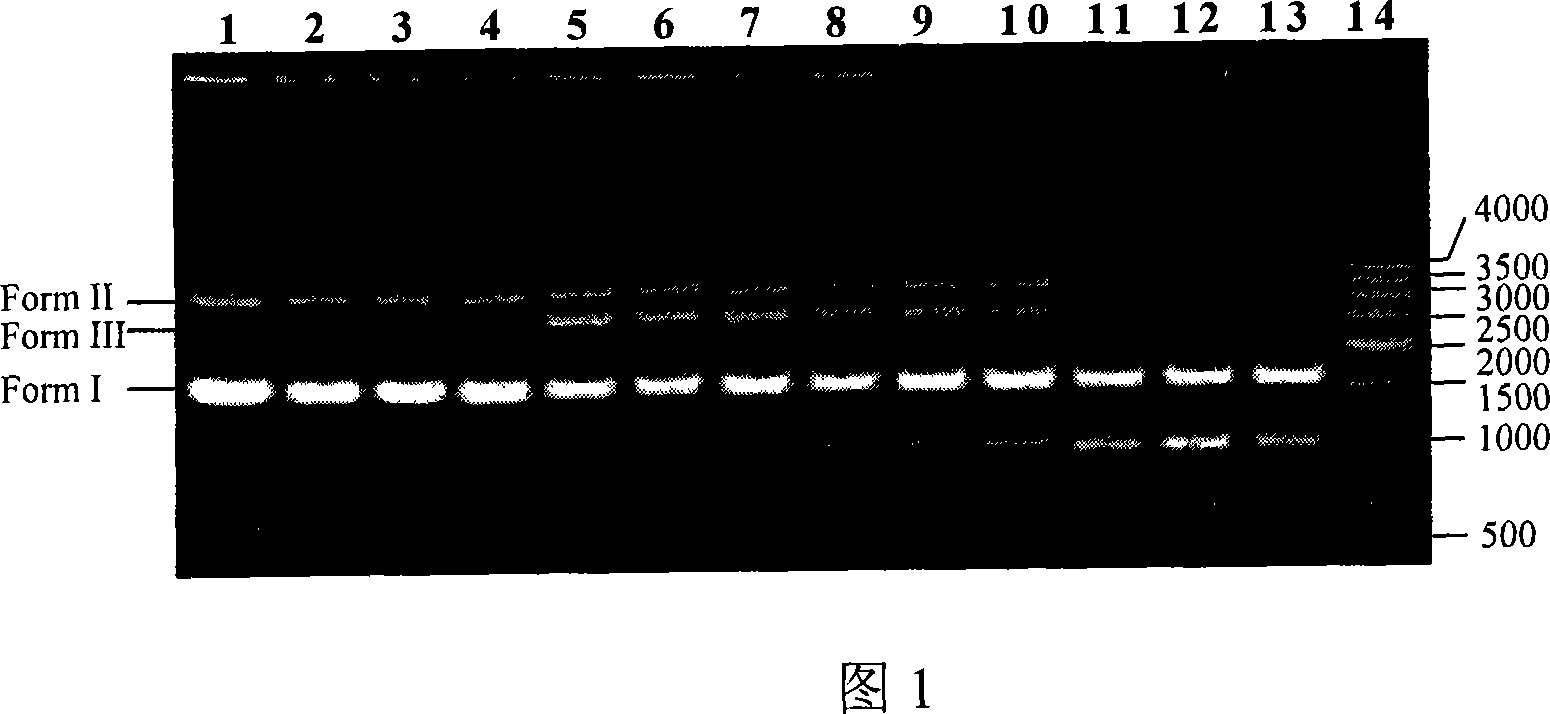
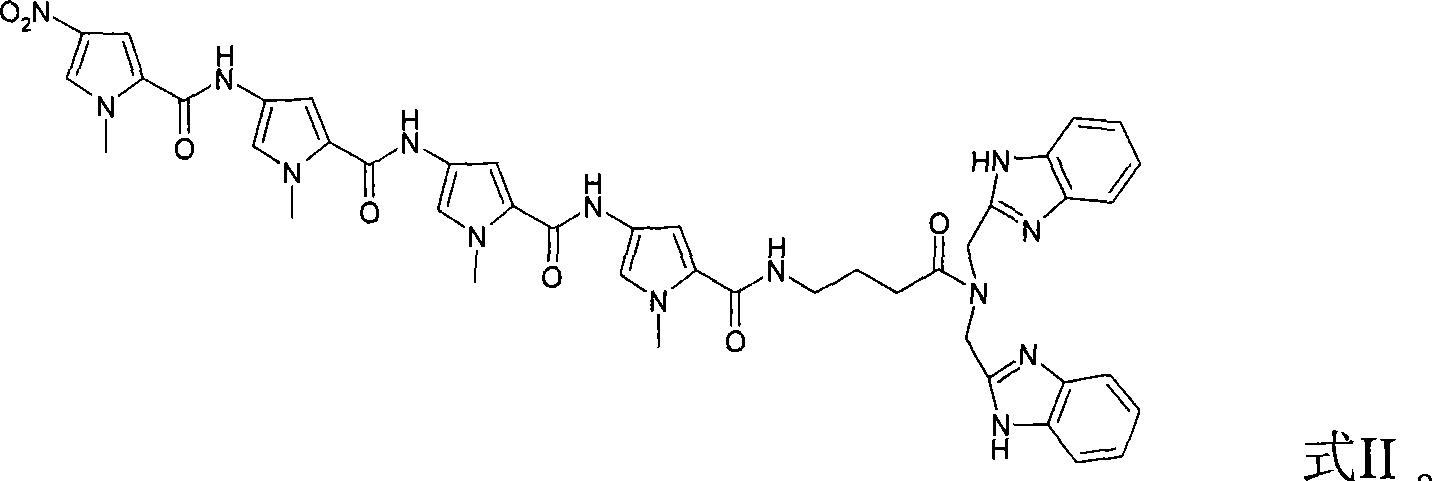
[0039] Example 2: N,N'-(benzimidazolyl)-λ-(4-(4-(4-(N-methylpyrrole-2-acyl)amino-N-methylpyrrole-2- Acyl)amino-N-methylpyrrole-2-acyl)amino-N-methylpyrrole-2-acyl)aminobutyramide and Zn 2+ The complex cleaves pUC18.
[0040] (1) N,N'-(benzimidazolyl)-λ-(4-(4-(4-(N-methylpyrrole-2-acyl)amino-N-methylpyrrole-2-acyl )Amino-N-methylpyrrole-2-acyl)amino-N-methylpyrrole-2-acyl)aminobutanamide and Zn 2+ Preparation of the complex
[0041] 1mmol N,N'-(benzimidazolyl)-λ-(4-(4-(4-(N-methylpyrrole-2-acyl)amino-N-methylpyrrole-2-acyl)amino -N-Methylpyrrole-2-acyl)amino-N-methylpyrrole-2-acyl)aminobutanamide and 1mmol zinc perchlorate hexahydrate (Zn(ClO 4 ) 2 ·6H 2 O) Dissolved in 15ml methanol, stirred at room temperature, reacted for 8 hours, a light yellow solid precipitated out, filtered under reduced pressure to obtain the solid, and dried.
[0042] (2) Cutting pUC18
[0043] N,N'-(benzimidazolyl)-λ-(4-(4-(4-(N-methylpyrrole-2-acyl)amino-N-methylpyrrole-2-acyl)amino -N-methylpyrrole-2-ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com