Method for prepring compound of beta hydroxyketone in supercritical liquid of carbon dioxide
A technology of carbon dioxide and compounds, applied in the field of preparation of β-hydroxy ketone compounds in supercritical carbon dioxide fluid, to achieve the effect of overcoming environmental pollution, reducing pollution, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
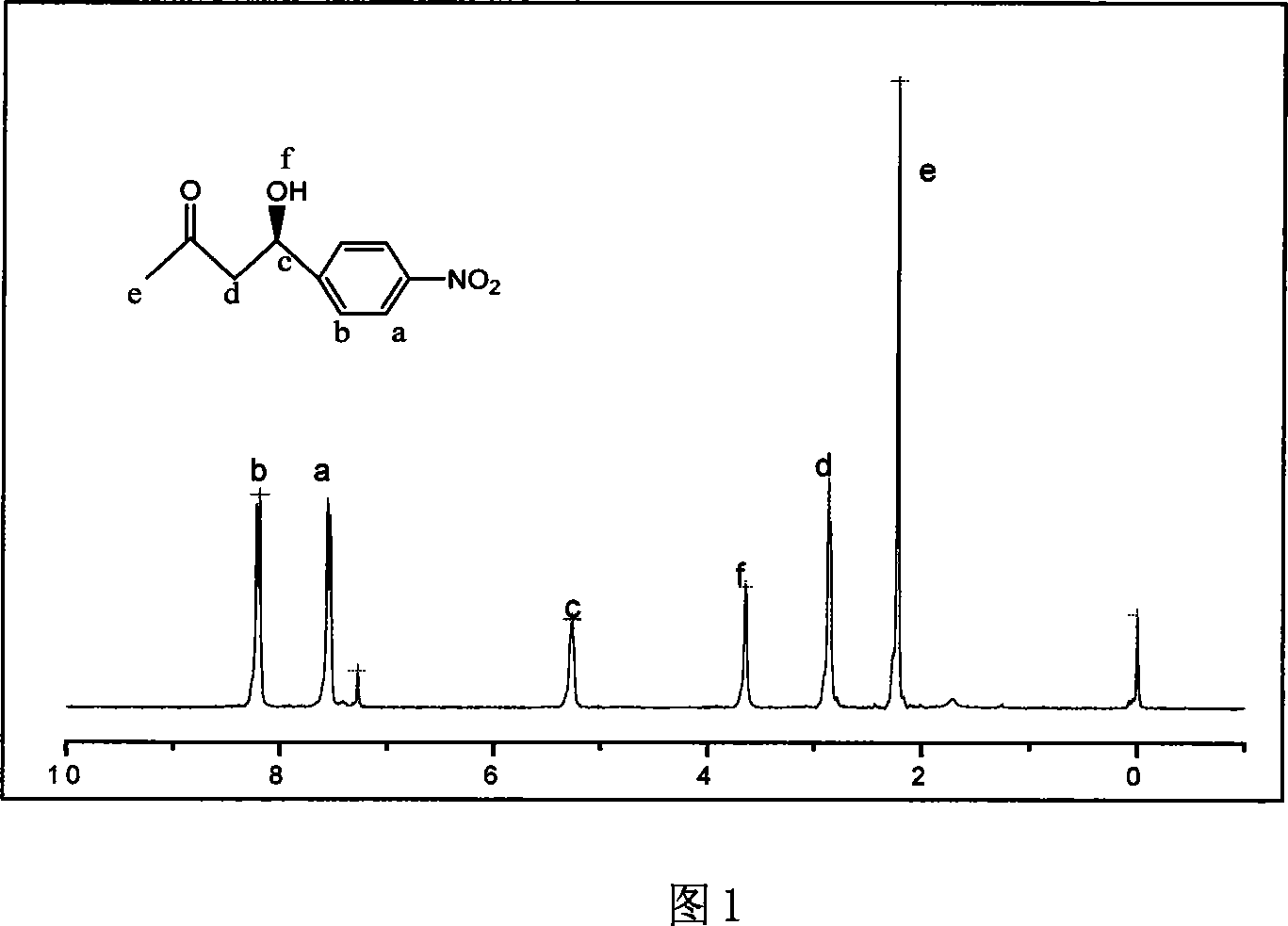
[0020] Taking the raw material p-nitrobenzaldehyde 0.5g used for the preparation of 4-(4-nitrophenyl)-4-hydroxyl-2-butanone as an example, other raw materials used and the process steps are as follows:
[0021] 1. Preparation of crude β-hydroxy ketone compound
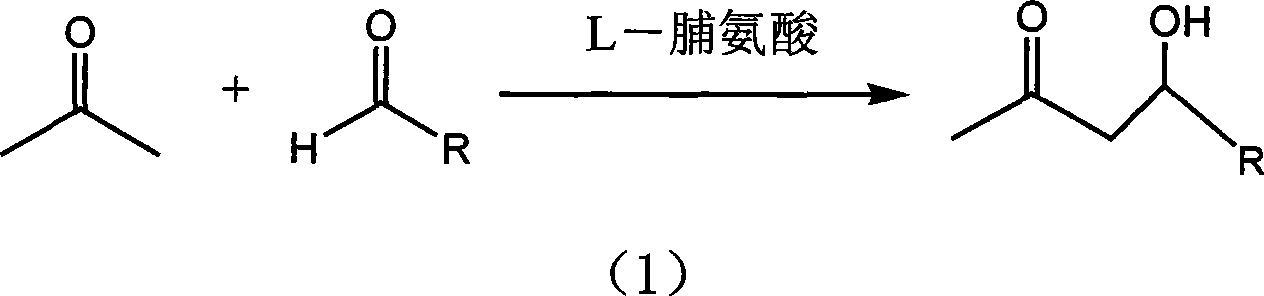
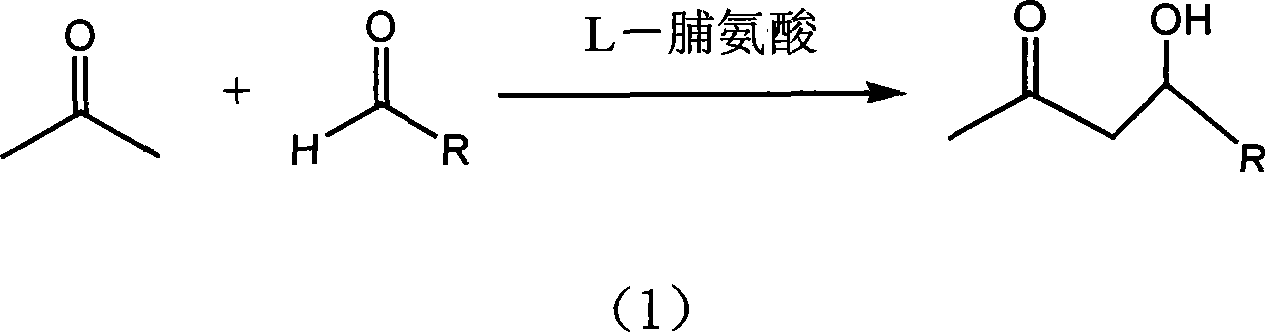
[0022] Add acetone 5.76g and p-nitrobenzaldehyde 0.5g in the supercritical autoclave, the molar ratio of p-nitrobenzaldehyde 0.5g and acetone is 1:30, add catalyst L-proline 0.057g, p-nitrobenzaldehyde The molar ratio of benzaldehyde to L-proline is 1:0.15, the reaction kettle is sealed, and CO is filled with a high-pressure micro-injection pump. 2 Fill the liquid to a pressure of 35MPa and react at 50°C for 30 hours. The chemical reaction equation is as follows:
[0023]
[0024] After the reaction is complete, CO is released 2 , add ethyl acetate to the reaction kettle until the reaction product dissolves, take out the solution, filter to remove the catalyst L-proline, and use a rotary evaporator to evaporate th...
Embodiment 2
[0028] Taking the raw material p-nitrobenzaldehyde 0.5g used for the preparation of 4-(4-nitrophenyl)-4-hydroxyl-2-butanone as an example, other raw materials used and the process steps are as follows:
[0029] In the preparation of β-hydroxy ketone compound crude product process step 1, add acetone 1.92g and p-nitrobenzaldehyde 0.5g in the supercritical autoclave, the mol ratio of p-nitrobenzaldehyde 0.5g and acetone is 1: 10, Add 0.114 g of catalyst L-proline, the molar ratio of p-nitrobenzaldehyde to L-proline is 1:0.3, seal the reaction kettle, and fill it with CO 2 The liquid was charged to a pressure of 20MPa, and reacted at 60°C for 15 hours. Other steps in this processing step are identical with embodiment 1.
[0030] Other steps are the same as in Example 1.
Embodiment 3
[0032] Taking the raw material p-nitrobenzaldehyde 0.5g used for the preparation of 4-(4-nitrophenyl)-4-hydroxyl-2-butanone as an example, other raw materials used and the process steps are as follows:
[0033] In the preparation of β-hydroxy ketone compound crude product process step 1, add acetone 3.84g and p-nitrobenzaldehyde 0.5g in the supercritical autoclave, the molar ratio of p-nitrobenzaldehyde and acetone is 1: 20, add catalyst L-proline 0.02g, the molar ratio of p-nitrobenzaldehyde and L-proline is 1: 0.05, seal the reaction kettle, fill with CO 2 The liquid was filled to a pressure of 10MPa, and reacted at 30°C for 36 hours. Other steps in this processing step are identical with embodiment 1.
[0034] Other steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com