Using methanol yeast to produce human kallikrein - 1
A kallikrein and methanol yeast technology, applied in the direction of enzymes, enzymes, hydrolytic enzymes, etc., can solve the problems of low yield, complicated purification process, and low effective yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
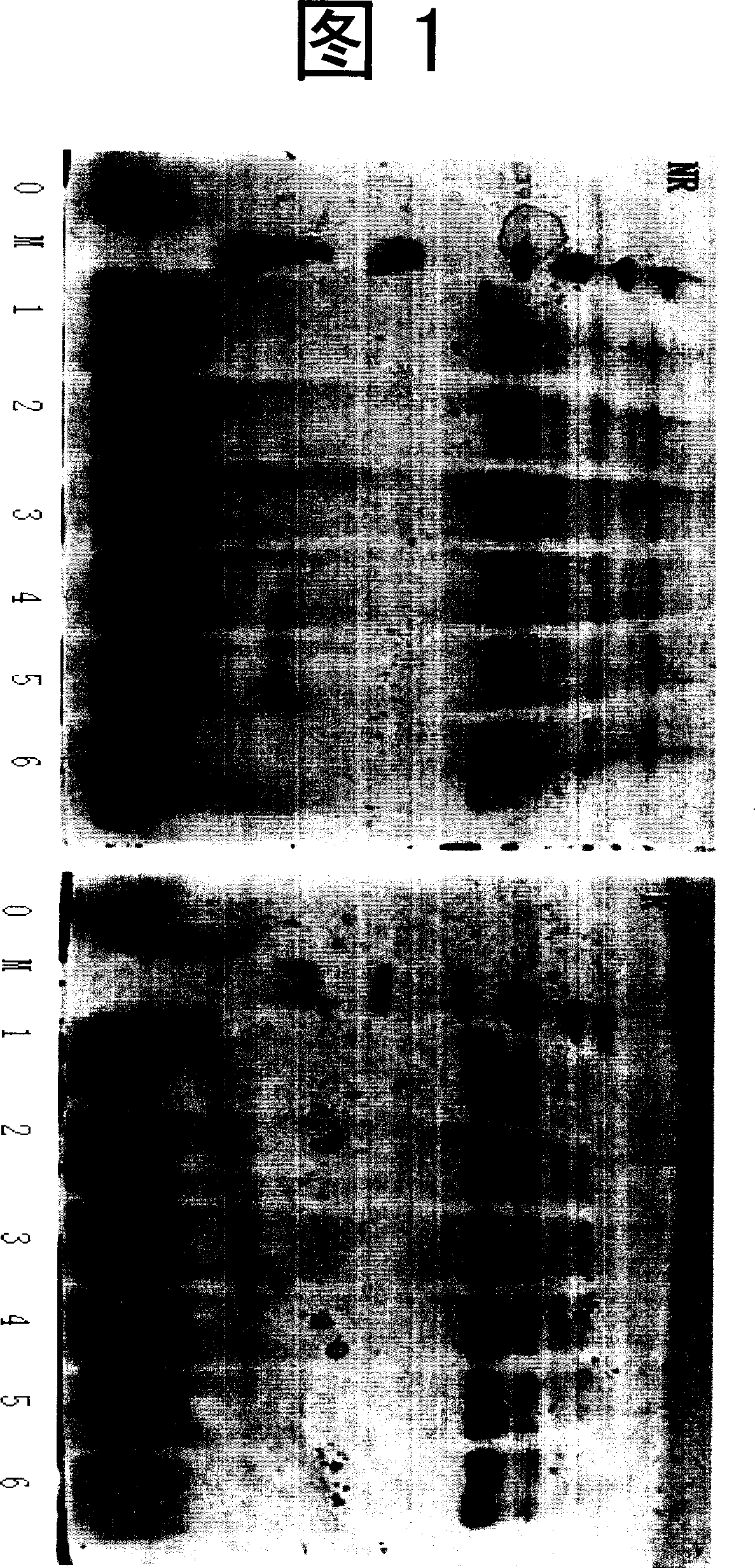
Image
Examples
Embodiment 1
[0048] k 1 Construction of engineered strains
[0049] 1. hK 1 Gene acquisition and expression vector pPICZa-hK 1 Construct
[0050] Refer to the relevant hK published in GeneBank 1 Complete gene sequence data, designed and synthesized (Shanghai Sangong) can amplify mature hK 1 Two primers of the gene Kal5 (5'-primer, SEQ-3) Kal3 (3'-primer, SEQ-4):
[0051] Kal5 (AA07624):
[0052] 5`-cat ctc gag aaa aga att gtg gga ggc tgg gag tgt gag-3`
[0053] Kal3 (AA07625):
[0054] 5`-cat gcg gcc gc t tag gag ttc tcc gct atg gtg tcc tc-3`
[0055] hK was obtained by PCR using the human kidney cDNA library (P / N: 7202, L / N: P0260602) from Panomics as a template 1 Gene. Primer Kal5 will be in hK 1 Add a DNA restriction enzyme XhoI site (CTCGAG) to the 5'-end of the gene, and the codon AAA AGA corresponding to the recognition sequence Lys-Arg of Kex2 protease, which will ensure that the inserted hK 1 When the gene is secreted and expressed, the α-signal peptide can be succe...
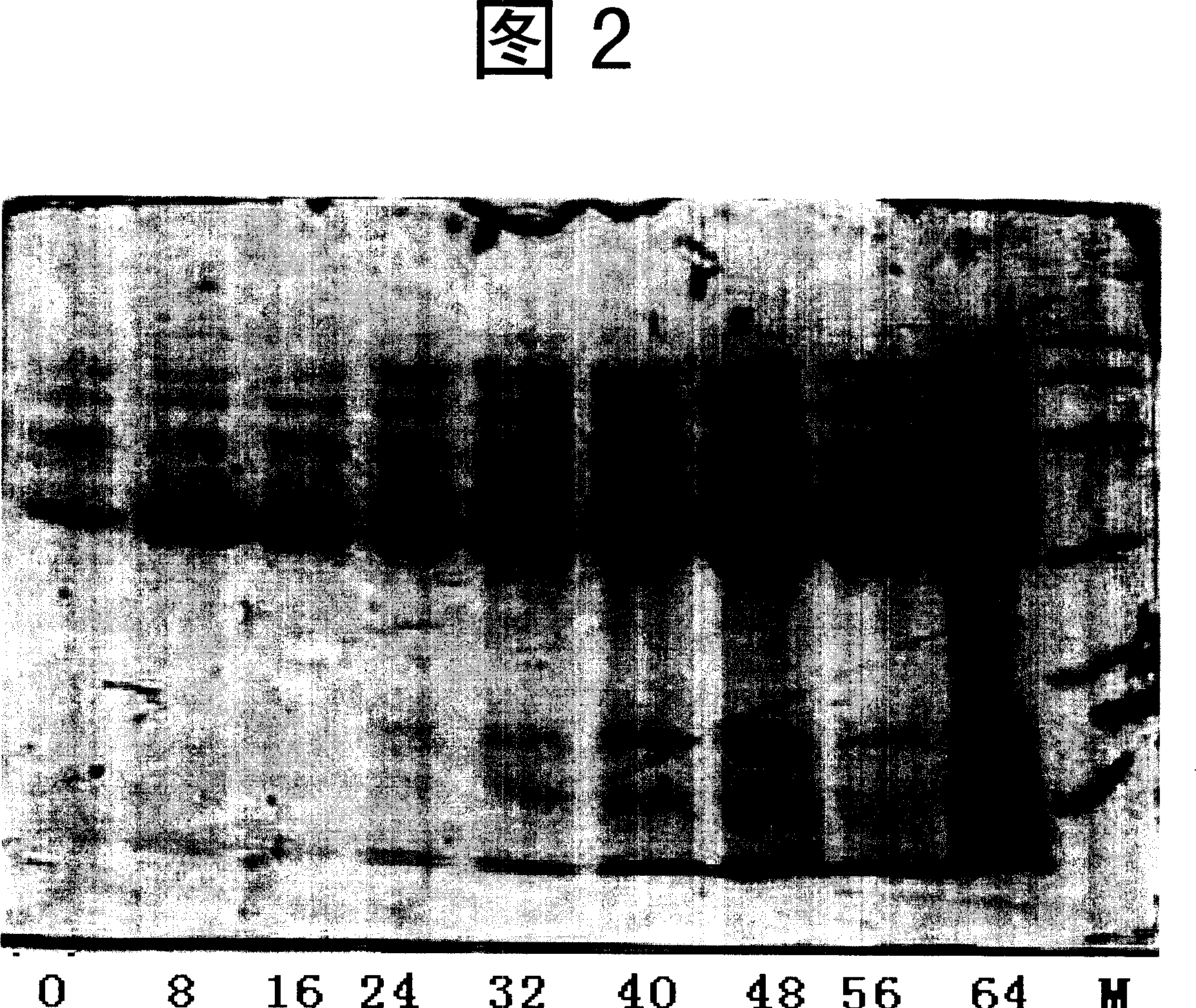
Embodiment 2
[0079] wxya 1 Fermentation of engineered bacteria
[0080] 1. Seed solution preparation
[0081] Take the working seed glycerol cryopreservation tube, after thawing, take 1ml and inoculate it into 500ml YPD medium (1% yeast powder, 2% peptone, 2% glucose), and cultivate in a shaker at 30°C and 300rpm for 30 hours to OD 600 If the value is 6.0±1.0, if the microscopic examination is normal, the seed solution can be used for inoculation. Preparation of basal salt medium BSM for fermentation 1 (K 2 SO 4 60.7 g, MgSO 4 24.2 g, CaSO 4 2H 2 O 3.9 g, H 3 PO 4 89ml, KOH 13.8g, PTM1 14ml, glycerol 400g, foam enemy 2ml, take 10L as an example, take the preparation of 1 liter of trace element medium PTM1 as an example, the content of each component is: CuSO 4 ·5H 2 O 6.0 g, NaI 0.008 g, MnSO 4 3.0 g, NaMoO 4 0.2 g, H 3 BO 3 0.02 g, ZnSO 4 20.0 g, CoCl 2 0.5 g, FeSO 4 ·7H 2 O 65.0 g, biotin 0.2 g, H 2 SO 4 5 ml, add water to make it 1 liter) and carry out solid...
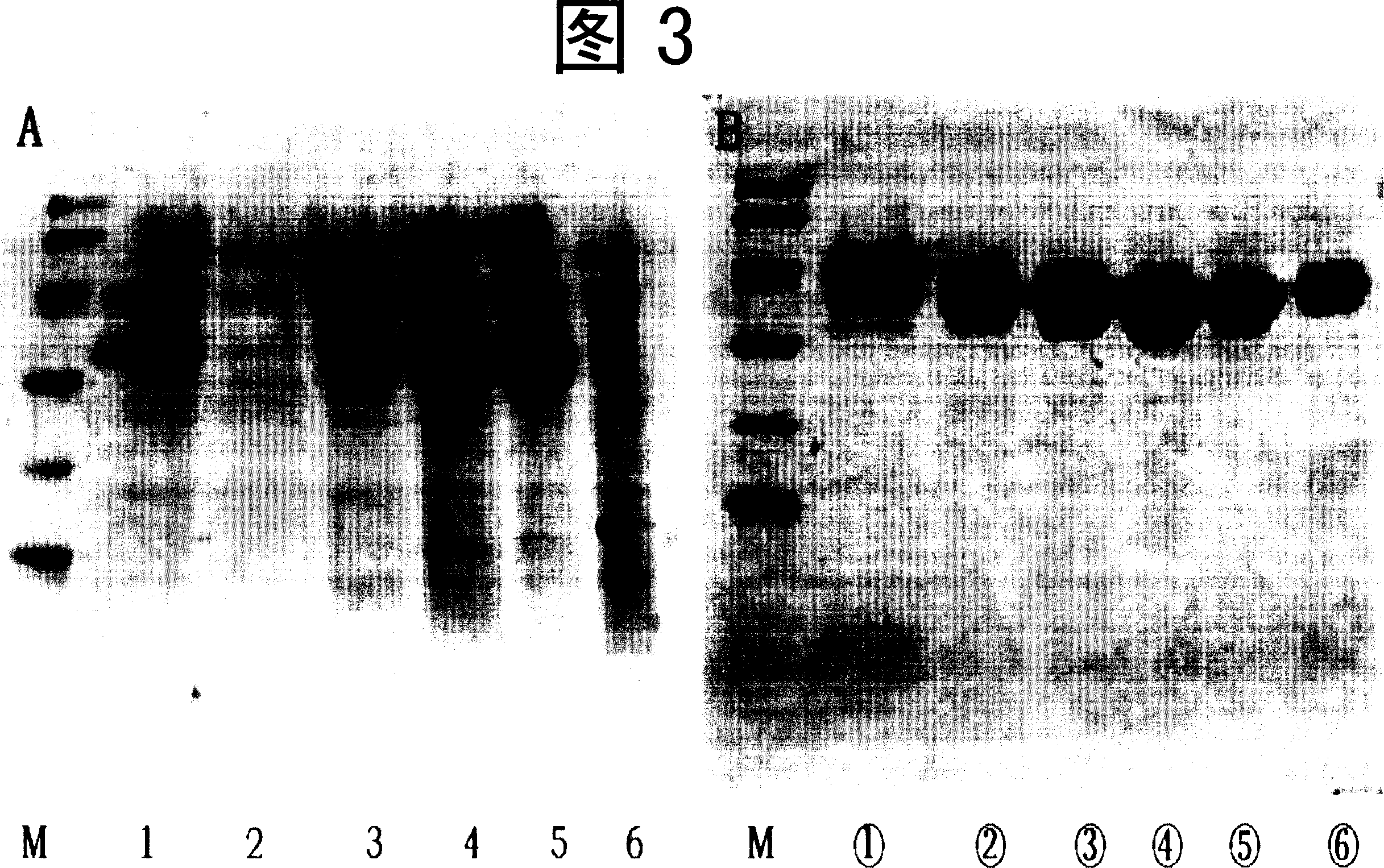
Embodiment 3
[0088] wxya 1 protein purification
[0089] The purification process consists of three steps: hydrophobicity, anion exchange and gel filtration chromatography, among which Phenyl-Sepharose FF is selected for hydrophobic chromatography, Q-Sepharose FF is used for anion exchange medium, and Superdex75 is used for gel filtration chromatography.
[0090] The specific process is as follows:
[0091] 1. Pretreatment of fermentation broth
[0092] Add 0.2M PB, pH 6.0 and 3.0M (NH 4 ) 2 SO 4Solution is respectively 20mM and 1.0M to its final concentration in the fermented liquid, adjusts the pH value to 6.0, leaves standstill 0.45 micron membrane filtration after 1 hour and contains 20mMPB, 1.0M (H 4 ) 2 SO 4 , pH6.0 treated fermentation liquid (sample liquid), just can carry out the chromatographic process.
[0093] 2. Phenyl-Sepharose FF hydrophobic chromatography
[0094] Phenyl Sepharose FF chromatographic column on the fermented liquid after above-mentioned treatment, co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com