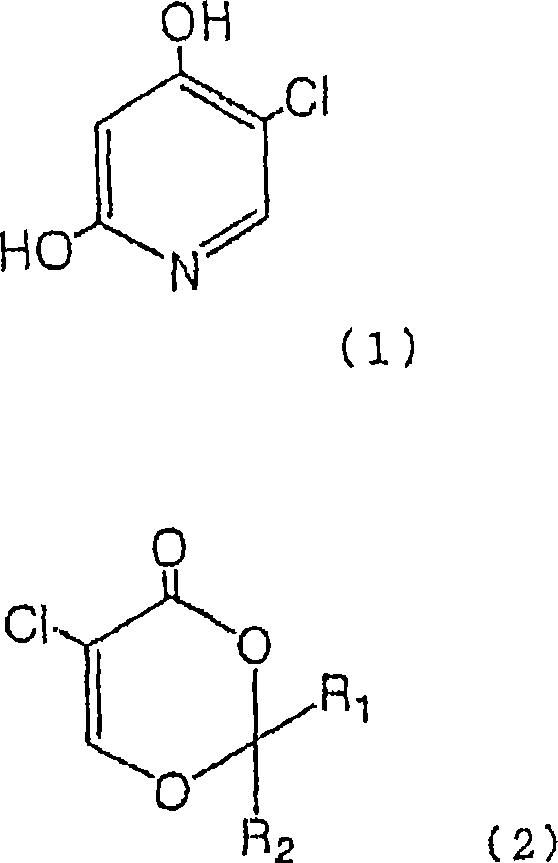
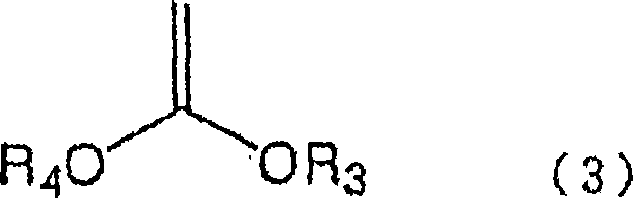
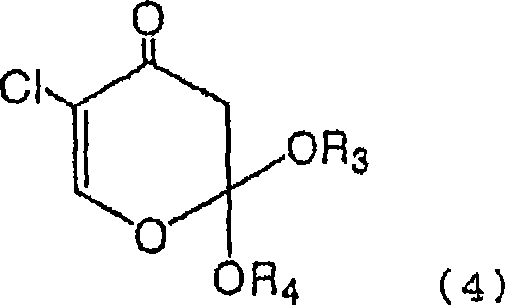
Process for production of 5-chloro-2,4-dihydroxypyridine
A technology of dihydroxypyridine and its manufacturing method, which is applied in the fields of chemical instruments and methods, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., can solve problems such as unfavorable environments, and achieve simple methods, mitigating conditions, and industrial The effect of less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0066] The method for producing 5-chloro-2,4-dihydroxypyridine (1) will be described in more detail by way of examples and reference examples, but the present invention is not limited to these examples.
reference example 1
[0068] Synthesis of 5-chloro-2,2-dimethyl-1,3-dioxin-4-one
[0069] Under ice-cooling, 2.44ml (0.03mol) of sulfonyl chloride was dropped into a solution of 3.2g (0.025mol) of 2,2-dimethyl-1,3-dioxin-4-one in pyridine (16ml). Stir at room temperature for 30 minutes and at room temperature for 2 hours. The reaction solution was ice-cooled, and water was added. The reaction solution was extracted with dichloromethane, dried over anhydrous sodium sulfate, and the dichloromethane layer was concentrated. The concentrated residue was purified by silica gel column chromatography (elution solvent n-hexane:ethyl acetate=95:5) to obtain 2.08 g of the title compound as an oily substance (yield 51.2%).
[0070] 1 H-NMR (CCl 4 )δ: 1.73(s, 6H), 7.23(s, 1H) MSm / z: 162(M + ), 164(M+2)
reference example 2
[0072] Synthesis of 5-chloro-2,2-cyclohexyl-1,3-dioxin-4-one
[0073] Under ice-cooling, 1.46ml (0.018mol) of sulfonyl chloride was dropped into a solution of 2.52g (0.015mol) of 2,2-cyclohexyl-1,3-dioxin-4-one in pyridine (13ml), and at the same temperature Stir at room temperature for 30 minutes and at room temperature for 2 hours. The reaction solution was ice-cooled, and water was added. The reaction solution was extracted with dichloromethane, dried over anhydrous sodium sulfate, and the dichloromethane layer was concentrated. The concentrated residue was purified by silica gel column chromatography (eluting solvent n-hexane:ethyl acetate=95:5), and the resulting oily substance was crystallized from n-hexane to obtain 2.00 g of the title compound (yield 65.9%). 1 H-NMR (CDCl 3 )δ: 1.21-2.21 (m, 10H), 7.28 (s, 1H)
[0074] MSm / z: 202(M + ), 204(M+2)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com