Method for manufacturing iron oxide with waste slag containing iron
A technology of iron oxide and waste residue, applied in the field of iron oxide, can solve the problems of difficulty in filtering and washing of hydrothermal products, and achieve the effects of being beneficial to cost, simple process and low temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
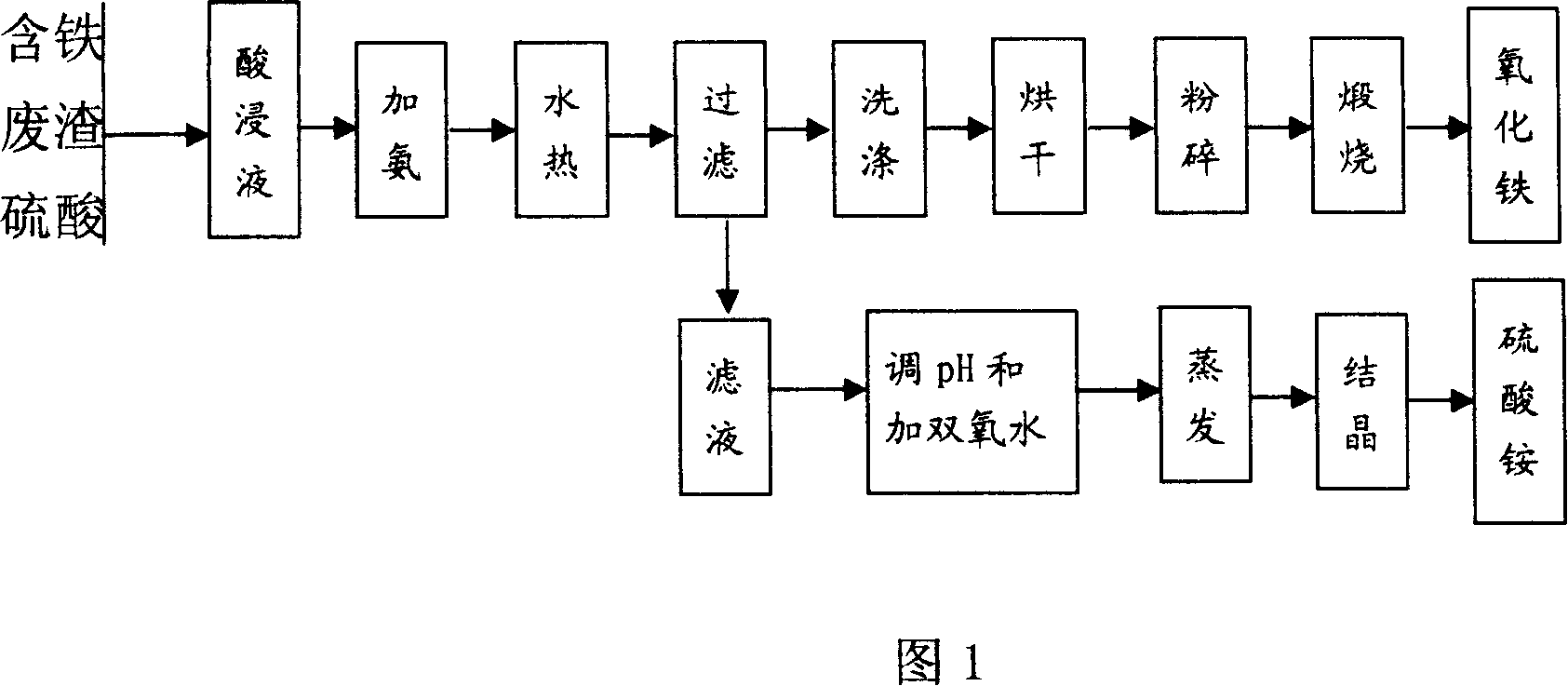
[0019] Embodiment 1: get pyrite slag 3000g, wherein slag contains Fe 2 o 3 : 74.1%, Fe 3 o 4 : 15.96%. Add 7740ml of 50% sulfuric acid in a 10L three-necked bottle, start stirring, and slowly add the above-mentioned slag. Control the reaction temperature to 115°C. After reacting for 4 hours, add an appropriate amount of water, and filter the obtained pickling solution while it is hot.
[0020] Take 10L pickling solution, adjust Fe in the pickling solution 3+ The concentration is 3.0mol / L, Fe 2+ / Fe 3 The molar ratio of the substances is 0.145, the adjusted pickling solution is added to the autoclave, 7200mL of ammonia water is added, and the pH of the reaction solution is controlled to be 7.0. Heating to 190°C and reacting for 2 hours, cooling, filtering, washing, and drying to obtain the crude iron oxide product. The crude iron oxide product was calcined at 800°C for 2 hours to obtain the iron oxide product, and the quality of the iron oxide product is shown in Table ...
Embodiment 2
[0026] Embodiment 2: get pyrite slag 300Kg, wherein slag contains Fe 2 o 3 : 74.1%, Fe 3 o 4 : 15.96%. at 1m 3 Add 50% sulfuric acid 0.774m to the enamel reaction kettle 3 , start stirring, and slowly add the above-mentioned slag. The reaction temperature is controlled to be 110° C., and after 4 hours of reaction, an appropriate amount of water is added, and the obtained pickling solution is filtered while it is hot.
[0027] Take 1m 3 Pickling solution, adjust Fe in the pickling solution 3+ The concentration is 3.2mol / L, Fe 2+ / Fe 3 The molar ratio of the substances is 0, and the adjusted acid leaching solution is added to the high-pressure reactor, and 0.72m 3 Ammonia, and control the pH of the reaction solution to be 7.5. Heating to 300°C for 2 hours, cooling, filtering, washing, and drying to obtain crude iron oxide. The crude iron oxide product was calcined at 800°C for 2 hours to obtain the iron oxide product, and the quality of the iron oxide product is show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com