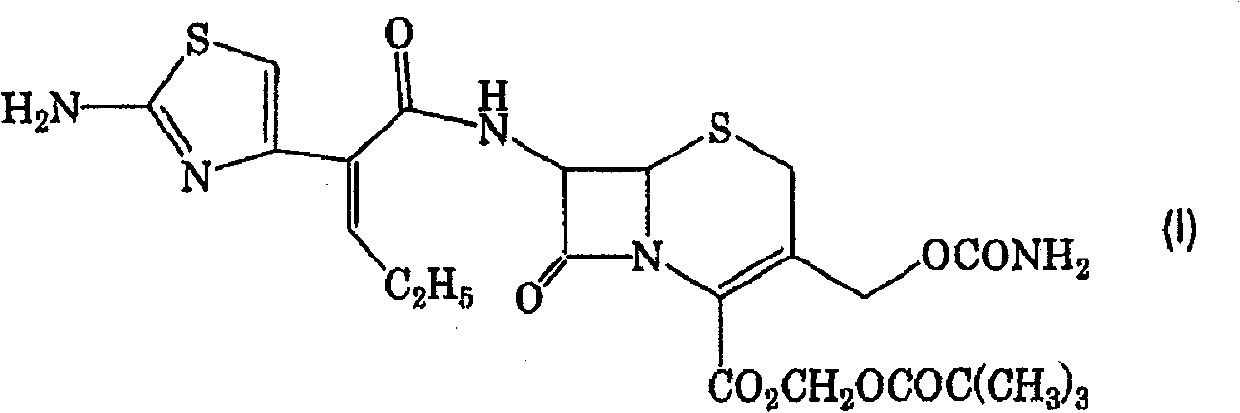
Cefcapene pivoxil methanesulfonate
A technology of cefcapene pivoxil and mesylate, which is applied in the fields of medical preparations containing active ingredients, antibacterial drugs, organic chemistry, etc., can solve the problems of cefcapene pivoxil mesylate and so on, and achieve high efficiency The effect of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] (1) Preparation of mesylate
[0031] The mesylate salts of the invention are preferably crystalline. The crystal may be a solvate, but is preferably an ansolvate. In the case of a solvate, examples of the solvent include water, alcohols (eg, methanol, ethanol), ketones (eg, methyl isobutyl ketone), and the like.
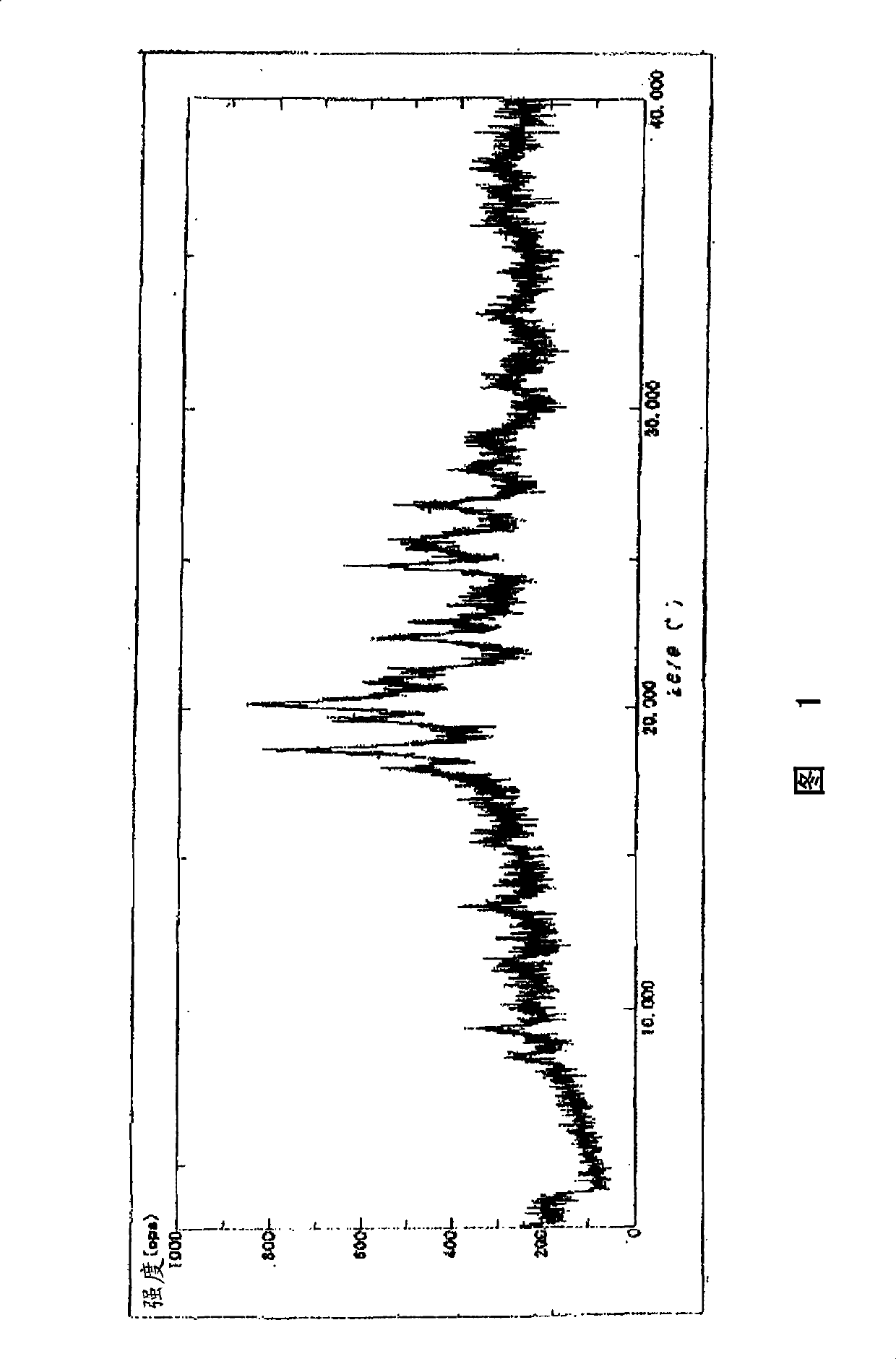
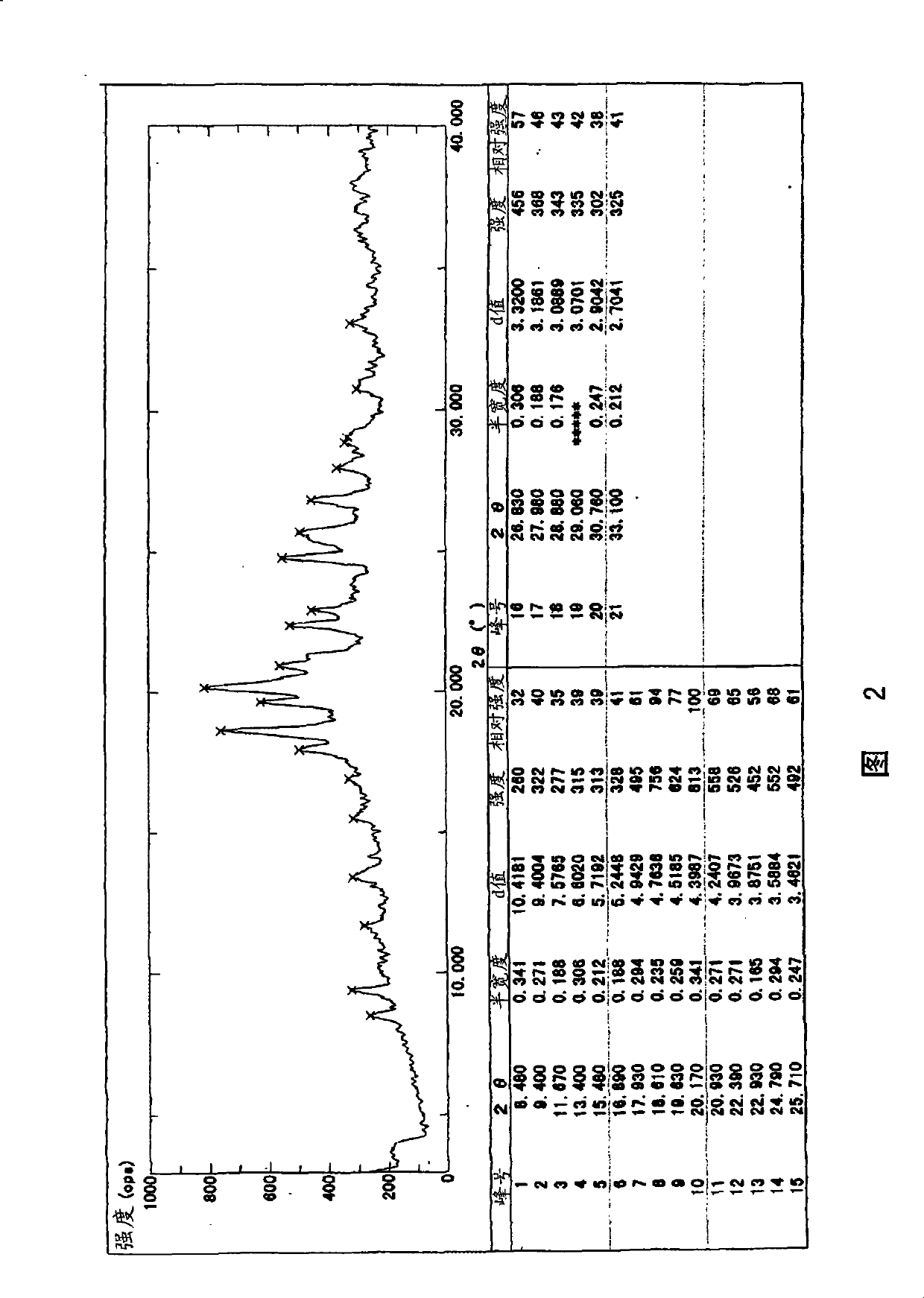
[0032] The crystal preferably shows the main peaks shown below by powder X-ray diffraction.
[0033] 2θ=17.93, 18.61, 19.63, 20.17, 20.93, 22.39, 24.79, 25.71 (unit: degree)
[0034] The method for producing the methanesulfonate is not particularly limited, but it is preferably produced from the corresponding hydrochloride. For example, methanesulfonic acid can be added to a solution containing cefcapene pivoxil hydrochloride in a solvent, preferably an organic solvent, to precipitate methanesulfonate or a solvate thereof, preferably crystals thereof.
[0035] Pure methanesulfonic acid may be used, or commercially available 70% methanesulfonic acid for ind...
Embodiment 1
[0054] Embodiment 1 (reclaims mesylate from mother washing liquor)
[0055] According to the method described in the preparation example 3 of the patent No. 2960790 bulletin (corresponding publication number: JP-P-4-295485), the 7-position amino protection body (7β-[(Z)-2-(2- tert-butoxycarbonylaminothiazol-4-yl)-2-butenoyl]amino-3-carbamoyloxymethyl-3-cephem-4-carboxylic acid pivaloyloxymethyl ester) 1039g Raw materials, under the condition that solvents and reagents are increased in proportion according to the amount of raw materials used, the same deprotection reaction is carried out to crystallize cefcapene axetil hydrochloride, filter, and collect crystals by filtration. The obtained crystals were washed successively with methyl isobutyl ketone and dichloromethane.
[0056] The mother lotion obtained by mixing 6.9 L of the filtrate (mother liquor) separated in the above-mentioned filtration process and 3.1 L of the washing liquor (wash liquor) separated after washing wit...
Embodiment 2
[0065] Embodiment 2 (hydrochlorination of mesylate)
[0066] Slurry 20.0 g of cefcapene pivoxil mesylate obtained in Example 1 in 140 mL of dichloromethane was added with 140 mL of ethanol water whose concentration was previously adjusted to 60.5%, and stirred and mixed. 5.9 g of a 20% aqueous sodium hydroxide solution was added thereto to neutralize and dissolve, and then the aqueous layer was removed by extraction. Then, it was extracted twice with 60 mL of 2.5% aqueous sodium chloride solution for washing to obtain an extract. In addition, the aqueous layer obtained during the extraction was back-extracted with 60 mL of dichloromethane, and the obtained back-extracted dichloromethane layer was combined with the extract. The combined extracts were concentrated under reduced pressure, and dichloromethane was distilled off to obtain about 48 g of an ethanol solution of cefcapene pivoxil. Dilute the obtained solution with 60 mL of methyl isobutyl ketone, drop it into a mixtur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com