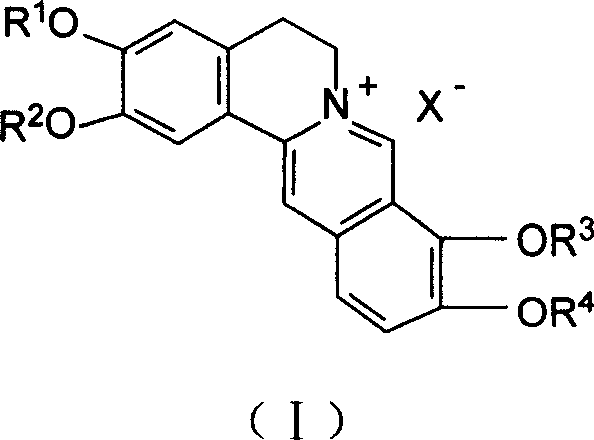
Aliphatic organic acid salt of berberine type alkaloid and preparation method and usage thereof
A technology of organic acid salts and alkaloids, which is applied in the field of pharmacy, can solve problems such as drug accumulation, and achieve the effects of improved bioavailability, good therapeutic effect, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of berberine laurate
[0038] Dissolve equivalent amounts of berberine sulfate and sodium laurate in 20 times the amount of 80℃ hot water, mix, continue stirring at 80℃ for 4 hours, let cool to room temperature, add the equivalent reactant (with berberine sulfate The reaction product was extracted three times with dichloromethane in an amount of 30 times the mass based on alkali and sodium laurate. The dichloromethane layer was separated and concentrated under reduced pressure. In the concentrated solution, 10 times the amount of petroleum ether was added to the filtrate to precipitate and filter. Take the precipitate, dry, and get it.
Embodiment 2
[0039] Example 2 Preparation of santengin stearate
[0040] Dissolve equivalent amounts of yellow rattan hydrochloride and sodium stearate in 10 times the amount of 90℃ hot water, mix, continue stirring at 90℃ for 4 hours, let cool to room temperature, add 20 times the amount of the reactant n-butyl The reaction product was extracted with alcohol three times, the n-butanol layer was separated, an appropriate amount of anhydrous sodium sulfate was added, shaken and stood still, filtered, the filtrate was taken and concentrated under reduced pressure, and 20 times the amount of petroleum ether was added to the concentrate to precipitate Precipitate, filter the precipitate, dry, and get it.
Embodiment 3
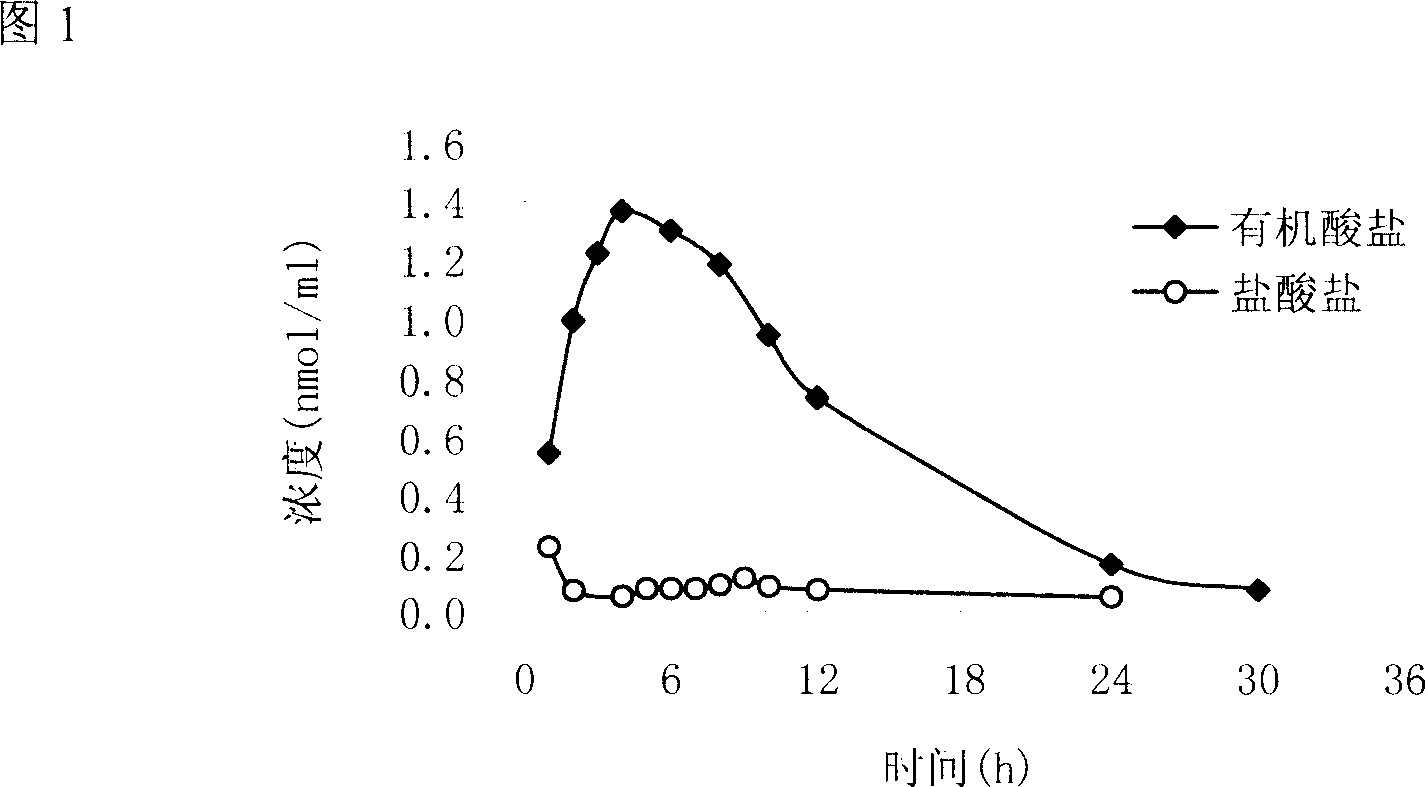
[0041] Example 3 Oral relative bioavailability of palmatine stearate.
[0042] Take the yellow rattan stearate prepared in Example 2 to make a solution with PEG 400, and give it to rats at a dose of 15 mg / kg. The same dose of yellow rattan hydrochloride PEG 400 solution was used as a control for comparison. Their pharmacokinetic curve characteristics and relative bioavailability. The pharmacokinetic curve shows that it has the advantages of good absorption and high bioavailability.
[0043] The classic two-compartment model after oral administration of yellow rattan stearate, the time lag is about 0.5h, the peak blood concentration is reached at 4-6h, and the elimination half-life is about 5.11h. Compared with santengin hydrochloride, the relative bioavailability of santengin stearate is 1855%, which does have the advantages of good absorption and high bioavailability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com