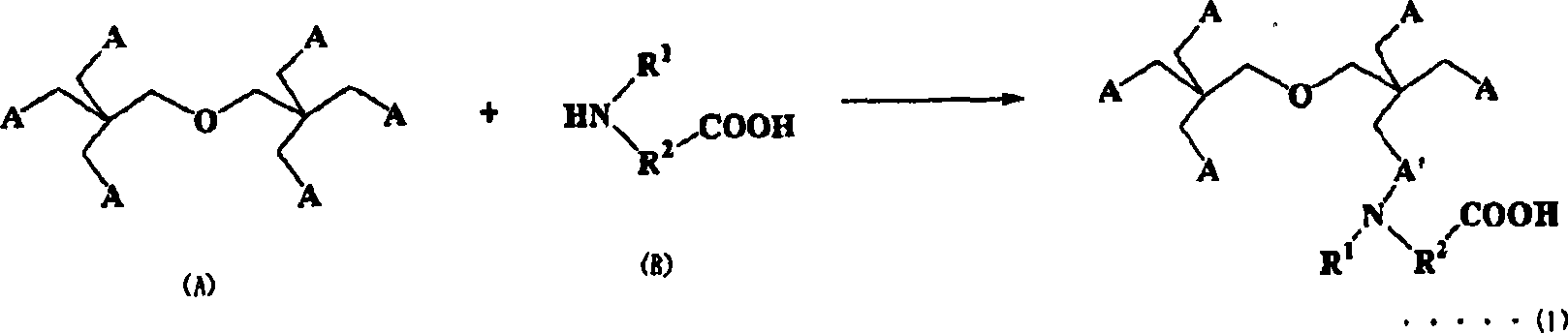
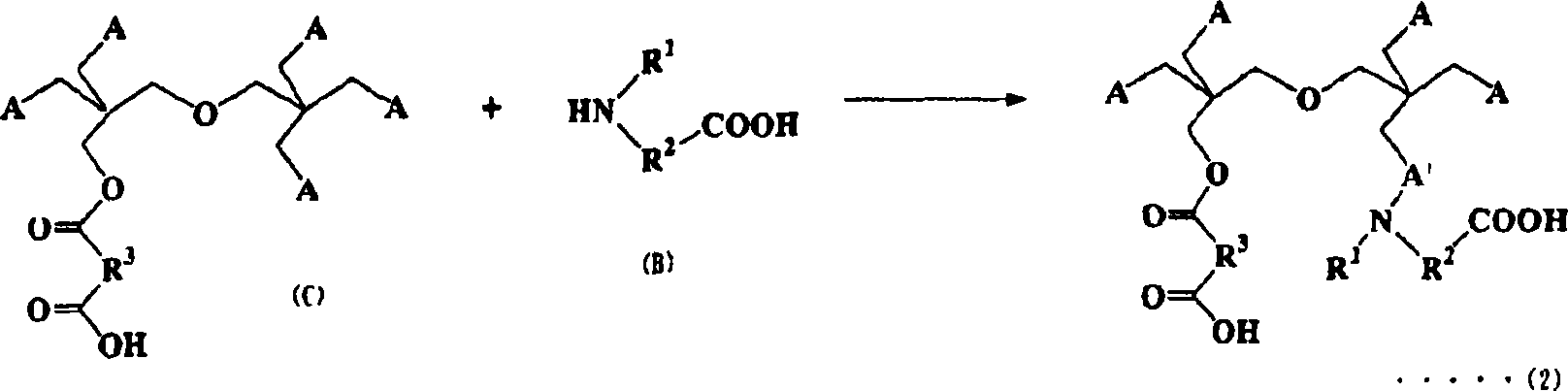
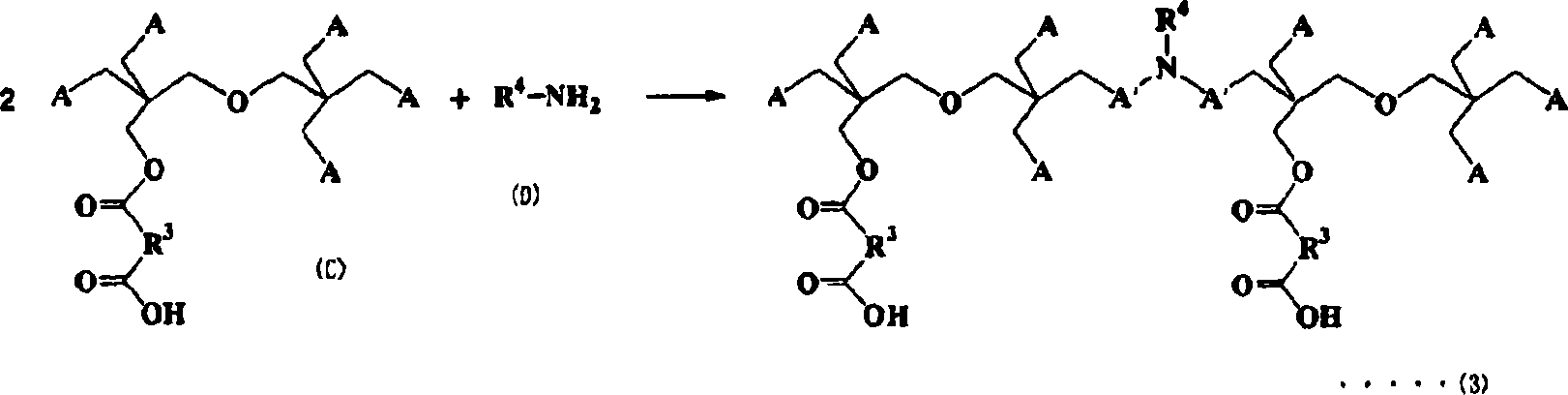
Compositions curable with actinic energy ray
A technology of active energy ray and composition, which is applied in the field of active energy ray curable composition, and can solve problems such as insufficient developability and insufficient sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0223] Examples and comparative examples are listed below to explain the present invention more specifically.
[0224] In addition, hereinafter, "parts" means parts by mass, and "%" means parts by mass.
manufacture example 1
[0225] ○Production Example 1 [Preparation of (a-1)]
[0226] In a 500 ml glass flask equipped with a stirring device, a thermometer, and a water-cooled condenser, 110 g of ethanol and 100 g of an acrylate mixture containing dipentaerythritol penta and hexaacrylate in a ratio of 30:70 (mass ratio) were added [hydroxyl value 36mgKOH / g, hereinafter referred to as "g1"], put 10 g of proline at room temperature and react for 4 hours. The reaction is carried out in an air atmosphere.
[0227] As a result, a solution containing 50% (a-1) (hereinafter referred to as a1) with an acid value of 45 mgKOH / g (converted as solid matter) as a solid matter was obtained.
manufacture example 2
[0228] ○Production Example 2 [Preparation of (a2-1)]
[0229] In the same flask as in Production Example 1, 16 g of succinic anhydride, 250 g of g1, and 0.13 g of mytol-hydroquinone complex were added, and the temperature was raised to 85°C. 1.3 g of triethylamine as a catalyst was put therein, and then reacted for 4 hours. The reaction was carried out in an air / nitrogen mixed atmosphere to obtain a compound having an acid value of 34 mgKOH / g (hereinafter referred to as "h1").
[0230] Next, add 103 g of DMDG [manufactured by Nippon Emulsifier Co., Ltd., trade name DMDG, hereafter referred to as "DMDG"], 100 g of g1, put 2.6 g of ethanolamine at room temperature, and react in an air atmosphere. 4 hours.
[0231] As a result, a solid substance containing 50% (a2-1) (hereinafter referred to as "a2") with an acid value of 33 mgKOH / g (converted to solids) and a hydroxyl value of 23 mgKOH / g (converted to solids) as solids Solution.
[0232] In addition, h1 was also used in Comparative ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com