Chiral dinaphtholsiloxane derivatives and preparation method thereof
A technology for binaphthol siloxane and derivatives, which is applied in the field of chiral binaphthol siloxane derivatives and their preparation, can solve the problems of weak bond and force between metals and ligands, poor catalytic reactivity and selectivity, and load The problem of small specific surface area of the catalyst, to achieve the effect of easy to obtain and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
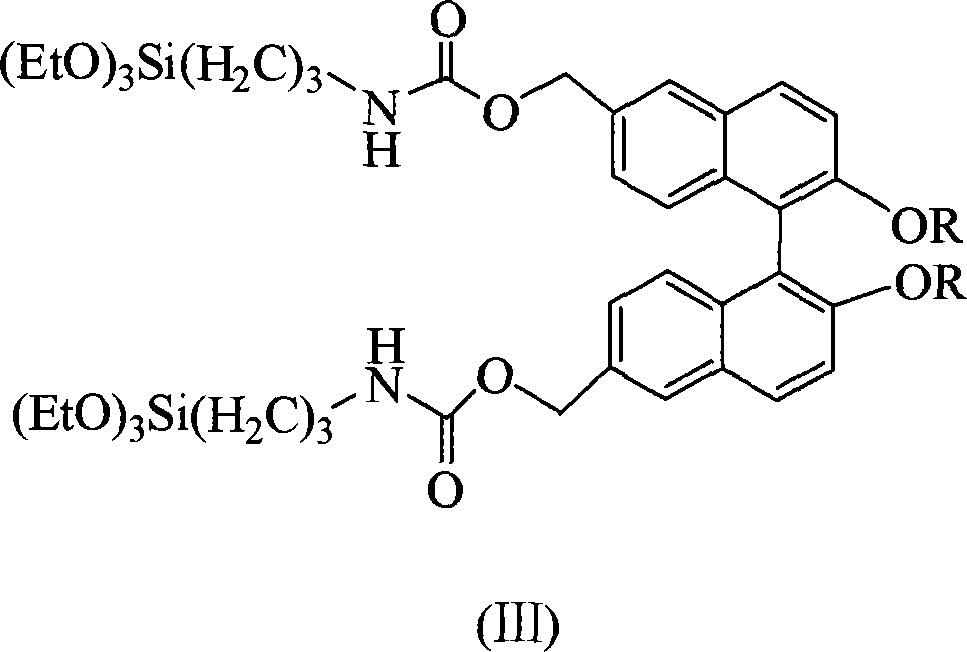
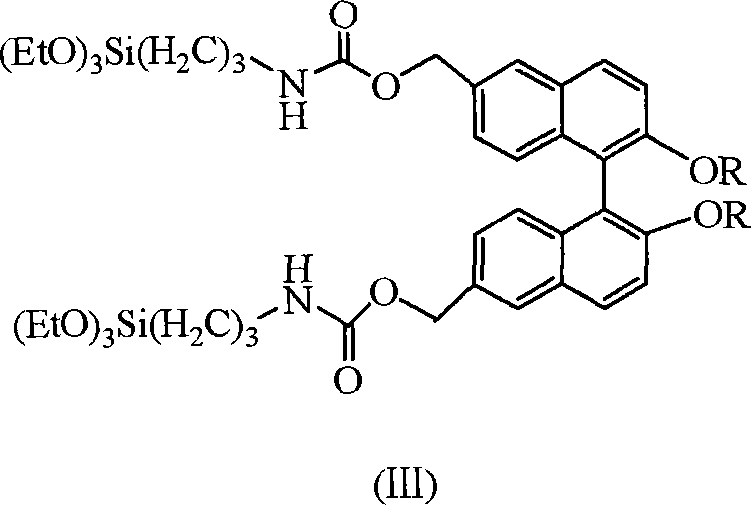
[0016] The general structural formula is (III), wherein, R is selected from -CH 2 OCH 3 Preparation of chiral binaphthol siloxane derivative 6,6'-bis(triethoxysilylpropylamino ester)methyl-2,2'-dimethoxymethoxybinaphthyl.
[0017] Compound (R)-6,6'-dimethylol-2,2'-dimethoxymethoxybinaphthyl 0.70g (1.60mmol), isocyanatopropyltriethoxysilane 1.58mL (6.40 mmol), triethylamine 0.91mL (6.40mmol) was dissolved in 15mL toluene, stirred under the protection of nitrogen, heated to reflux for 24 hours, cooled, filtered to remove insoluble matter, the filtrate was distilled under reduced pressure to remove toluene, and used petroleum ether: ethyl acetate = 2:1 as the eluent, separated by flash column chromatography to obtain 1.11 g of the target product as a white solid, with a yield of 74.5%. It was proved by NMR and elemental analysis that it was indeed the target compound:
[0018] 1 H NMR (CDCl 3 )δ: 8.06 (s, 2H), 7.83-7.85 (d, 2H), 7.40-7.44 (d, 2H), 7.32-7.34 (d, 2H), 7.20-7.2...
Embodiment 2
[0020] The general structural formula is (III), wherein, R is selected from -CH 3 Preparation of chiral binaphthol siloxane derivative 6,6'-bis(triethoxysilylpropylamino ester)methyl-2,2'-dimethoxybinaphthyl.
[0021] Compound (R)-6,6'-dimethylol-2,2'-dimethoxybinaphthyl 0.60g (1.60mmol), isocyanatopropyltriethoxysilane 1.58mL (6.40mmol), Triethylamine 0.91mL (6.40mmol) was dissolved in 15mL toluene, stirred under nitrogen protection, heated to reflux for 26 hours, cooled, filtered to remove insoluble matter, and the filtrate was distilled under reduced pressure to remove toluene, using petroleum ether: ethyl acetate = 5: 1 was used as the eluent to separate by flash column chromatography to obtain 1.09 g of the target product as a white solid, with a yield of 78.5%. It was proved by NMR and elemental analysis that it was indeed the target compound:
[0022] 1 HNMR (CDCl 3 )δ: 8.02 (s, 2H), 7.80-7.82 (d, 2H), 7.40-7.44 (d, 2H), 7.31-7.33 (d, 2H), 7.19-7.22 (d, 2H), 6.13-6....
Embodiment 3
[0024] The general structural formula is (III), wherein, R is selected from-C 12 h 25 Preparation of chiral binaphthol siloxane derivative 6,6'-bis(triethoxysilylpropylamino ester)methyl-2,2'-bis(dodecyloxy)binaphthyl.
[0025]Compound (R)-6,6'-dimethylol 2,2'-bis(dodecyloxy)binaphthyl 0.82g (1.20mmol), isocyanatopropyl triethoxysilane 1.48mL (6.00 mmol), triethylamine 0.85mL (6.00mmol) was dissolved in 15mL toluene, stirred under the protection of nitrogen, heated to reflux for 30 hours, cooled, filtered to remove insoluble matter, the filtrate was distilled under reduced pressure to remove toluene, and used petroleum ether: ethyl acetate =3:1 as the eluent, separated by flash column chromatography to obtain 0.95 g of the target product as a colorless oil, with a yield of 66.3%. It was proved by NMR and elemental analysis that it was indeed the target compound:
[0026] 1 HNMR (CDCl 3 )δ: 8.04 (s, 2H), 7.82-7.84 (d, 2H), 7.41-7.45 (d, 2H), 7.33-7.35 (d, 2H), 7.21-7.24 (d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com