Method for preparing polythene derivative in supercritical fluid
A technology of supercritical fluid and polyethylene, which is applied in the production of bulk chemicals to achieve the effects of reducing loss, reducing molecular weight distribution, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Taking the raw material trifluoroethyl methacrylate 10.214g (0.0608 mole) used for preparing polytrifluoroethyl methacrylate as an example, other raw materials used and the preparation process steps are as follows:
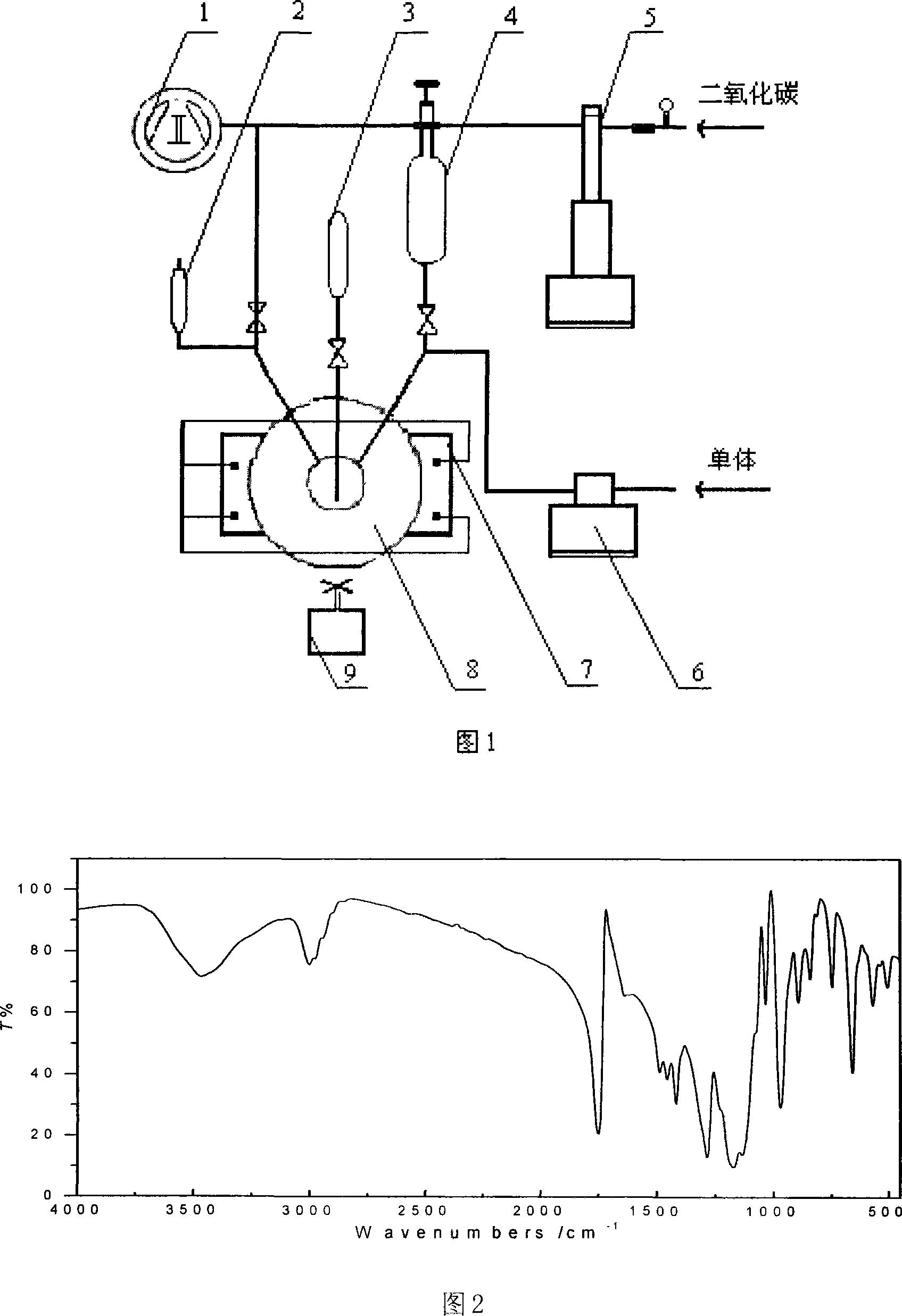
[0031] 1. Vacuumize the reactor
[0032] Charge CO into the reaction kettle 8 with a syringe pump 5 at room temperature 2 The pressure of the gas to the reactor 8 is 0.5MPa, release the charged gas, vacuumize the reactor 8 with a vacuum pump 1 to make the vacuum degree in the reactor 8 0.09MPa, repeat the above steps 2 times. It is also possible to fill the reactor with N 2 gas, Ar gas can also be filled, and N in the reactor can be filled 2 Or the pressure of Ar gas and the vacuum degree after the reactor is evacuated and filled with CO 2 Same for gas.
[0033] 2. Polymerization reaction
[0034] In this embodiment, the monomer is trifluoroethyl methacrylate, the free radical initiator is azobisisobutyronitrile, and the fluid is CO 2 . 10.214 g of t...
Embodiment 2
[0044] Taking the raw material trifluoroethyl methacrylate 10.214g (0.0608 mole) used for preparing polytrifluoroethyl methacrylate as an example, other raw materials used and the preparation process steps are as follows:
[0045] In polymerization step 2, trifluoroethyl methacrylate is selected as the monomer in this embodiment, azobisisobutyronitrile is selected as the free radical initiator, and CO is selected as the fluid. 2 . 10.214 g of trifluoroethyl methacrylate is added to the reaction kettle 8 with the metering pump 6, and the CO is charged into the reaction kettle 8 with the syringe pump 5 2 To the pressure in the reactor 8 is 6.9MPa, trifluoroethyl methacrylate and CO in the reactor 8 2 The volume of the fluid is 20%~30% of the volume of the reactor 8, and the reactor 8 is warmed up to 47.5°C with the heater 7, and 0.0099 g of azobisisobutyronitrile is added to the reactor 8 through the sample tube 4 and the syringe pump 5, The molar ratio of trifluoroethyl metha...
Embodiment 3
[0051] Taking the raw material trifluoroethyl methacrylate 10.214g (0.0608 mole) used for preparing polytrifluoroethyl methacrylate as an example, other raw materials used and the preparation process steps are as follows:
[0052] In polymerization step 2, trifluoroethyl methacrylate is selected as the monomer in this embodiment, azobisisobutyronitrile is selected as the free radical initiator, and CO is selected as the fluid. 2 . 10.214 g of trifluoroethyl methacrylate is added to the reactor 8 with the metering pump 6, and CO is charged into the reactor 8 with the syringe pump 5 until the pressure in the reactor 8 is 6.7 MPa, and the methacrylic acid in the reactor 8 is Trifluoroethyl ester and CO 2 The volume of the fluid is 20%~30% of the volume of the reactor 8, and the reactor 8 is warmed up to 70° C. with the heater 7, and 0.223 g of azobisisobutyronitrile is added to the reactor 8 through the sample tube 4 with the syringe pump 5, The molar ratio of trifluoroethyl me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com