M-crystal system of adefovir dipivoxil ester and preparation method and medicine application
A technology of adefovir dipivoxil and crystal form, which is applied in chemical instruments and methods, medical preparations containing active ingredients, compounds of elements of Group 5/15 of the periodic table, etc., can solve the problems of high cost, harsh conditions, and preparation Cumbersome methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1. M crystal form adefovir dipivoxil and its preparation
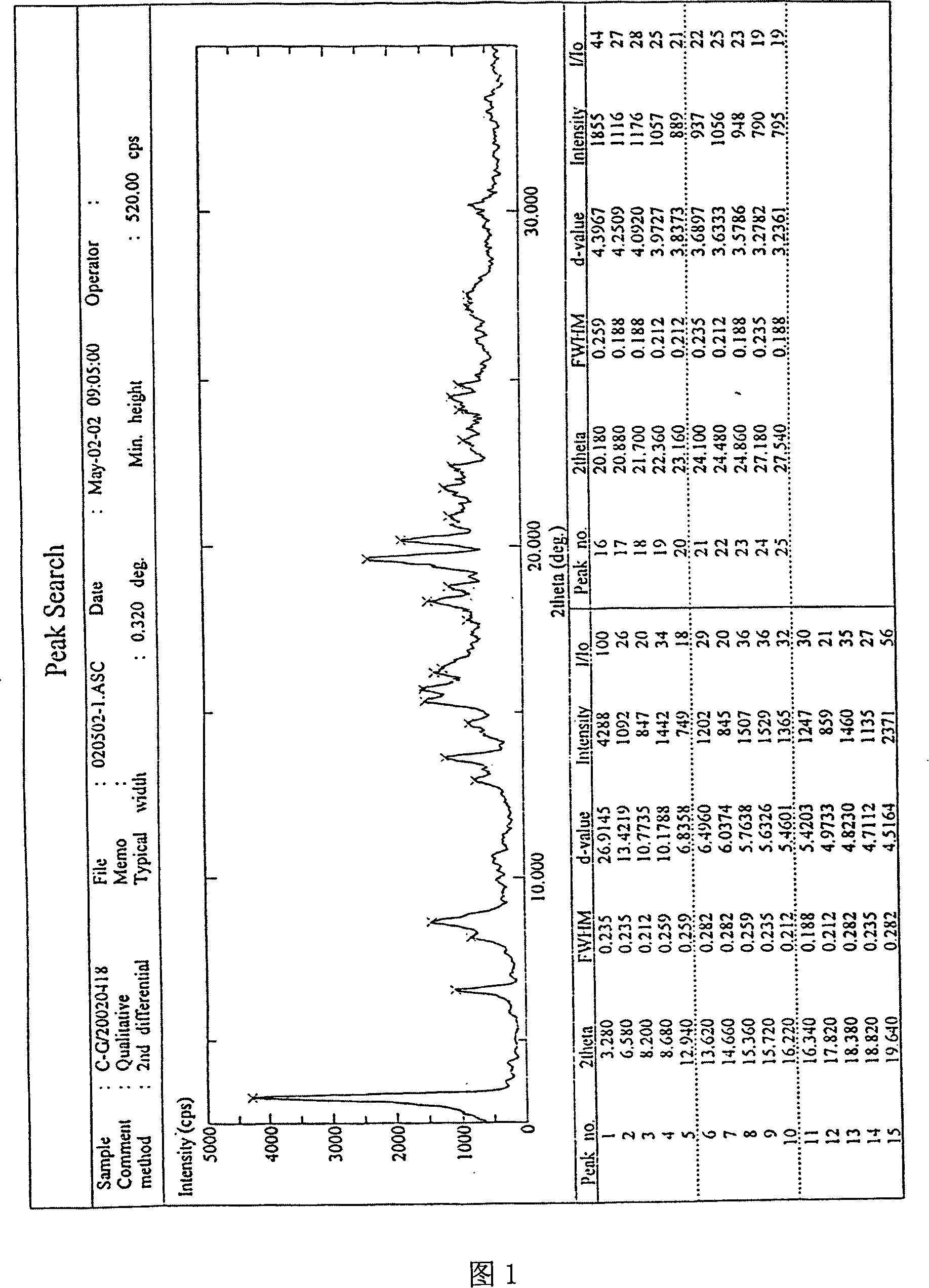
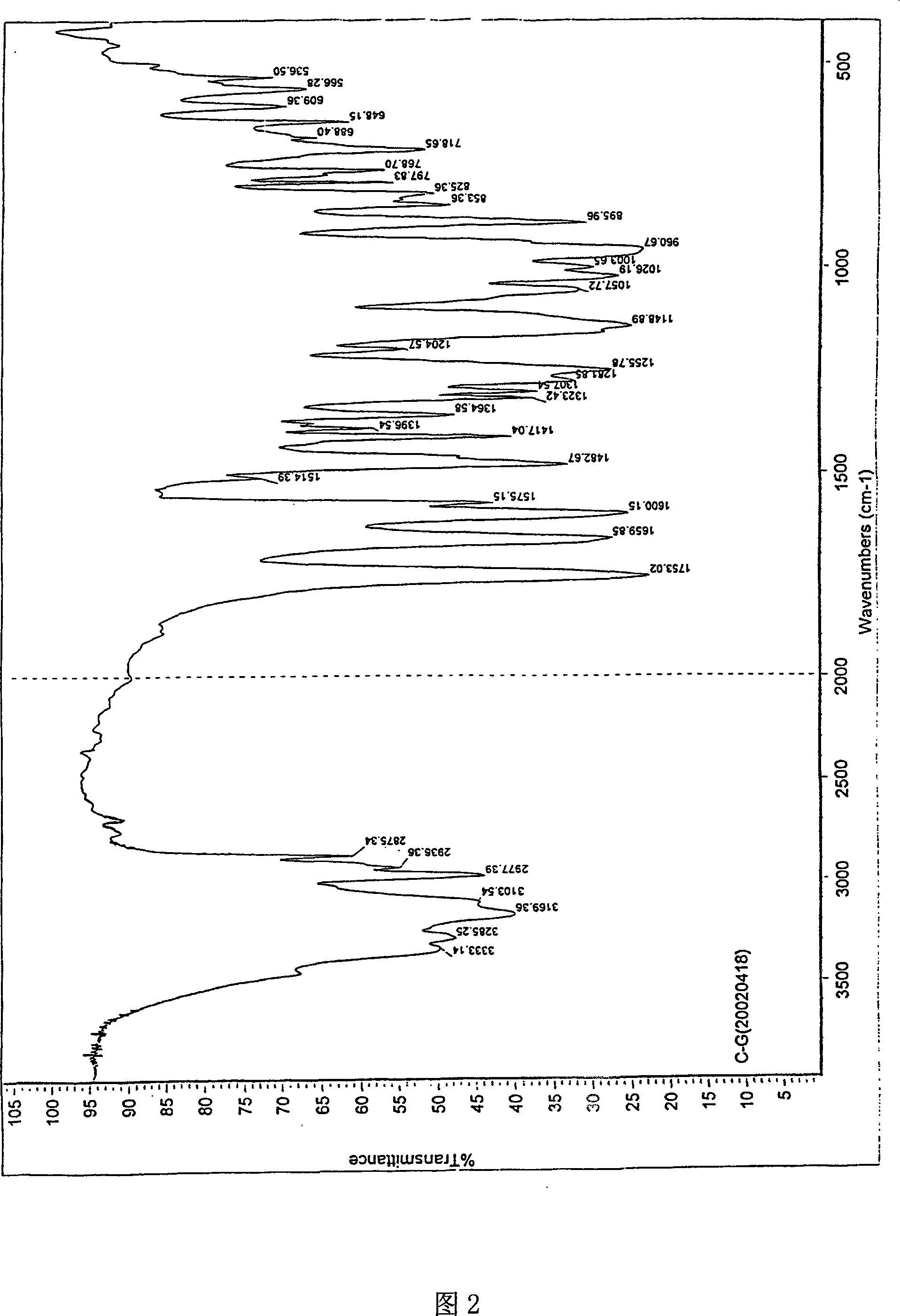
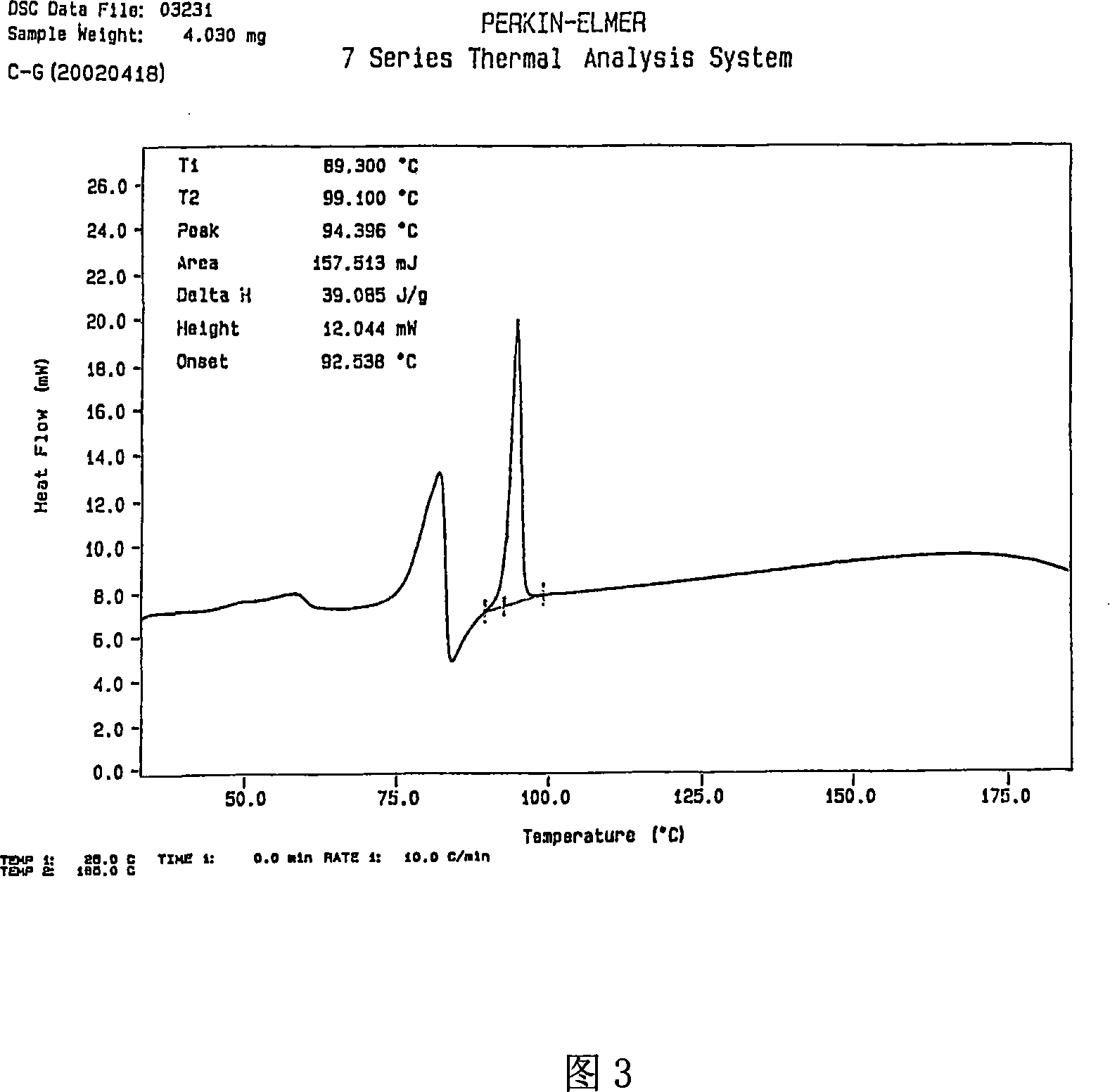
[0033] Take 5g of adefovir dipivoxil, dissolve it in 25ml of dichloromethane, put it in an ice-water bath at 10°C, concentrate it in a rotary evaporator for 20min under reduced pressure, spin dry the dichloromethane to obtain a solidified product, scrape out the solid, and wash it with 5ml of petroleum ether , at P 2 o 5 In an existing vacuum desiccator, vacuum-dry at room temperature for more than 8 hours to obtain the M crystal form of adefovir dipivoxil of the present invention. Its X-ray diffraction diagram, infrared absorption spectrum diagram and thermal difference analysis diagram are consistent with the accompanying drawings.
Embodiment 2
[0034] Embodiment 2. Tablet containing M crystal form adefovir dipivoxil and its preparation
[0035] Prescription: M crystal form adefovir dipivoxil 10g, hydroxypropyl cellulose 100g, sodium carboxymethyl starch 30g.
[0036] Preparation method: the above two materials were crushed through 100 mesh respectively, dried under reduced pressure at 100°C for more than 10 hours, cooled to room temperature, mixed evenly with M crystal form adefovir dipivoxil, granulated by dry granulator, and pressed into 1000 Tablets, that is, tablets of M crystal form adefovir dipivoxil, each containing 10 mg of M crystal form adefovir dipivoxil.
Embodiment 3
[0037] Example 3. Capsules containing M crystal form adefovir dipivoxil and its preparation
[0038] Prescription: M crystal form adefovir dipivoxil 5g, lactose 50g, microcrystalline cellulose 65g, sodium carboxymethyl starch 20g, magnesium stearate 1g.
[0039] Preparation method: the above-mentioned lactose, microcrystalline cellulose, and sodium carboxymethyl starch were respectively crushed through 100 meshes, dried under reduced pressure at 100°C for more than 10 hours, cooled to room temperature, and mixed evenly with M crystal form adefovir dipivoxil. Granulate by dry granulation machine, add magnesium stearate, mix well, and fill into 1000 empty capsules to obtain the capsules of M crystal form adefovir dipivoxil, each capsule contains M crystal form adefovir dipivoxil 5 mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com