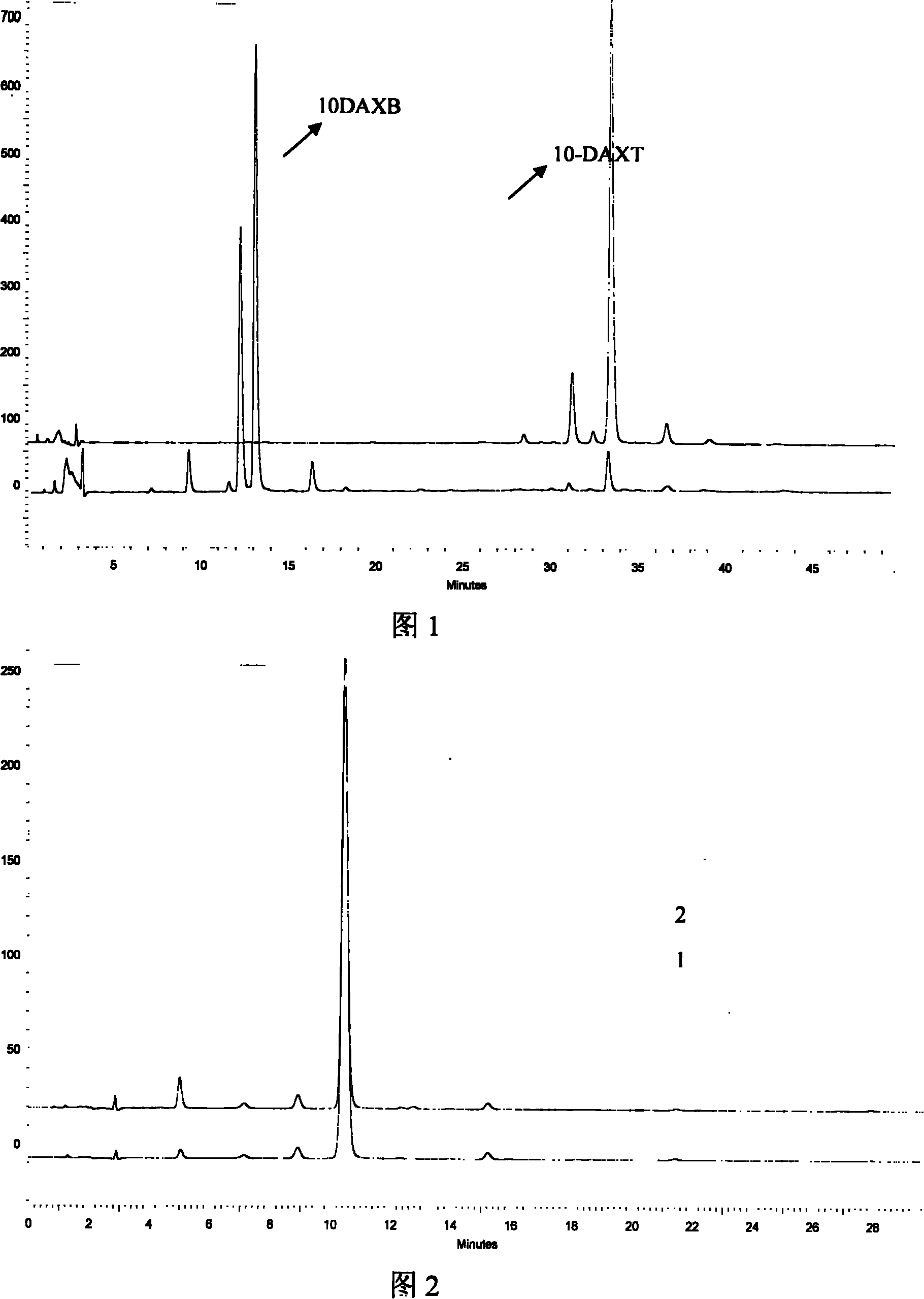
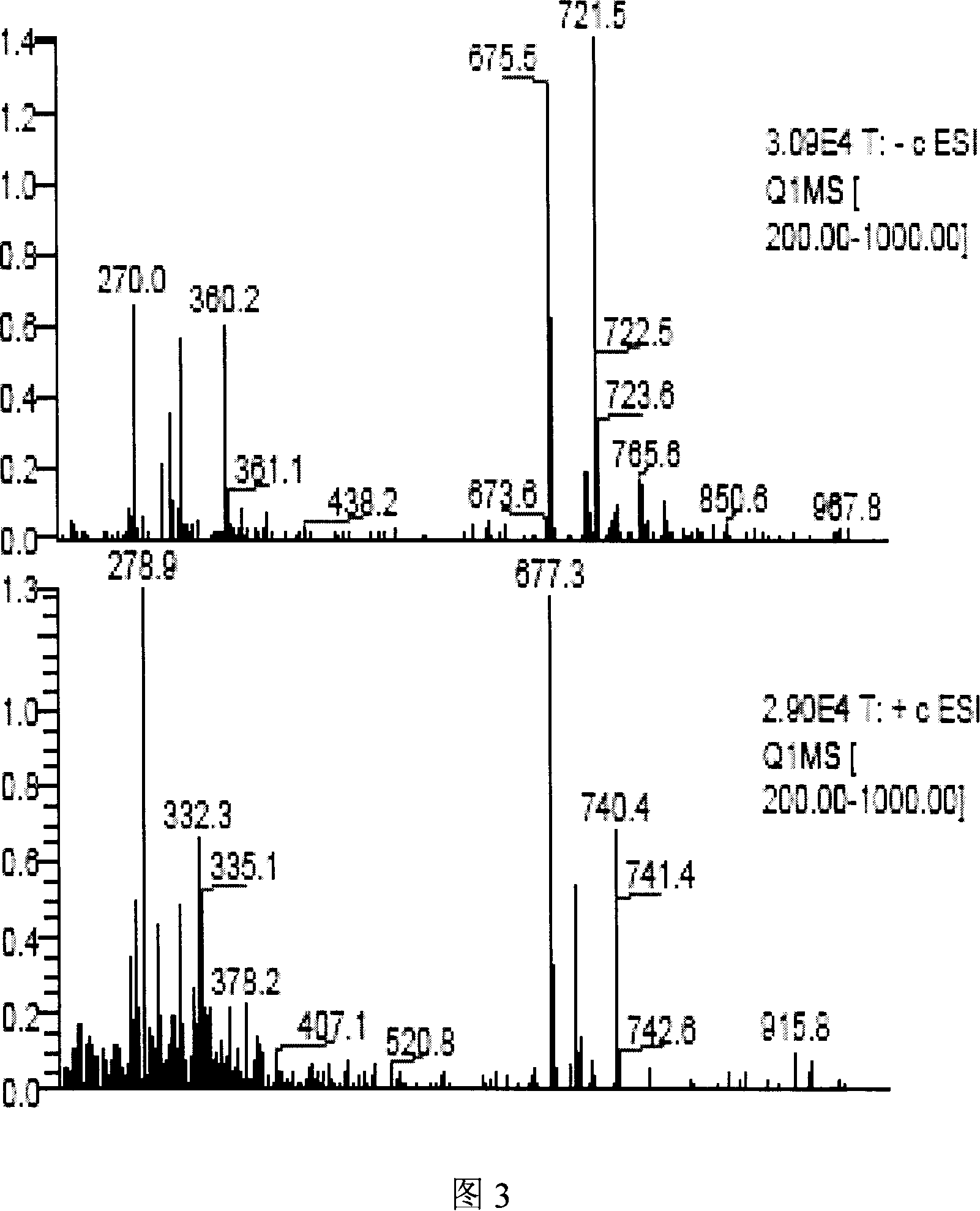
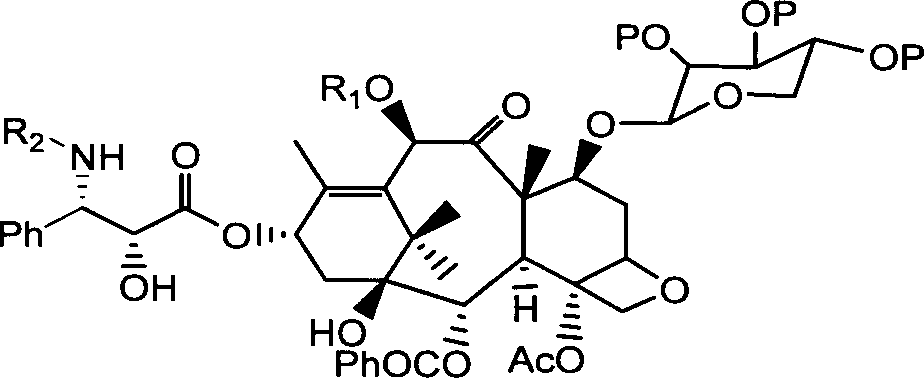
Preparation of 10-deacetyl 7-xylose baccatin or 7-xylose baccatin
A technology for removing acetylation and xylose, which is applied in the fields of sugar derivatives, bulk chemical production, organic chemistry, etc., to achieve the effects of simple process, wide application range and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Conversion of 10-DAXT to 10-DAXB
[0043] 1.0 g of 7-xylose taxane mixture containing 10-deacetyl 7-xylose paclitaxel (51%) was dissolved in 10 ml of THF, and 0.36 g of NaBH was added 4 , after stirring for 2 hours, the reactant was poured into 50% acetic acid-water solution, stirred for 10 minutes, and NaBH that did not participate in the reaction was removed 4 , and extracted three times with ethyl acetate, the organic phases were combined and dried over anhydrous sodium sulfate for 6 hours. After filtration, it was concentrated to obtain 1.12 g of crude product with a 10-DAXB content of 54%.
Embodiment 210-D
[0044] Purification of embodiment 2.10-DAXB
[0045] Pack 15 grams of silica gel into a column (column diameter 1.9 cm, column bed height 9.5 cm), dissolve 1.12 grams of 10-DAXB crude product in dichloromethane; use 90% dichloromethane / methanol as eluent, elute 800 ml and then TLC After testing and concentrating the same components, 0.47 g of 85% 10-DAXB was obtained with a yield of 112% (the raw material contains other xylose taxanes). Dissolve 0.47 g of 85% 10-DAXB in 40 ml of hot ethanol, add 5 ml of triple-distilled water dropwise under ultrasound; leave it at room temperature for 6 hours, then move it to the refrigerator for 24 hours and filter to obtain 0.33 g of white crystals. The purity was determined by HPLC 95%, the mother liquor continues to crystallize to obtain high-purity 10-DAXB:
Embodiment 3
[0046] Example 3: Conversion of 10-DAXC to 10-DAXB
[0047] 0.10 g of 10-deacetyl 7-xylose cephalomannine (91%) was dissolved in 2 ml of THF, and 0.04 g of (Me) 4 NBH 4 , stirred at room temperature for 2 hours. After the reaction, the reactant was poured into cold glacial acetic acid-water solution and stirred for 10 minutes to remove NaBH that did not participate in the reaction. 4 . Extracted 3 times with ethyl acetate, combined the organic phases and dried over anhydrous sodium sulfate for 6 hours. After filtration and concentration, 0.11 g of 87% 10-DAXB was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com