Catalyst for removing oxygen and method for removing oxygen using the catalyst
A technology of catalysts and oxides, applied in the direction of catalyst activation/preparation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of catalyst thermal degradation, performance degradation, and oxygen removal performance may not be able to say Sufficient and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061]
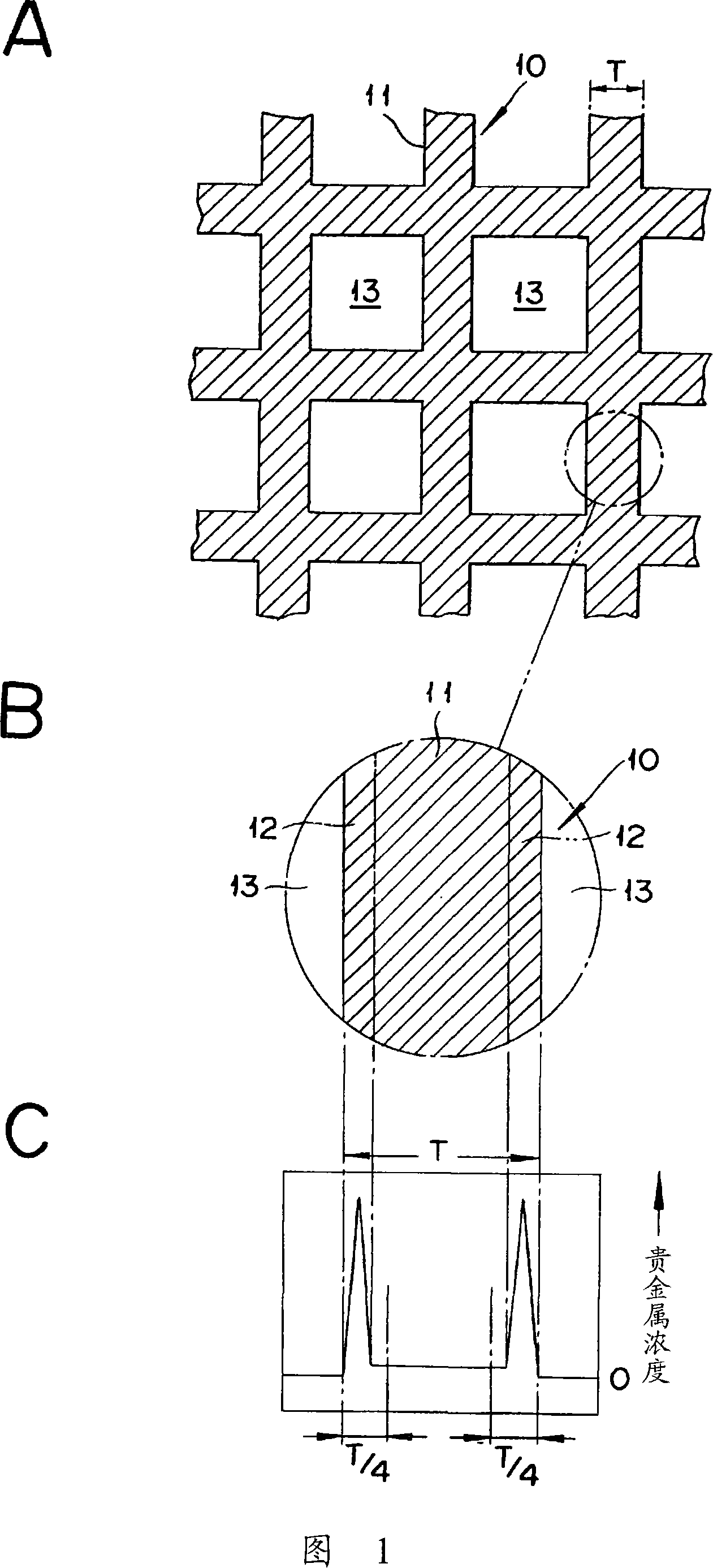
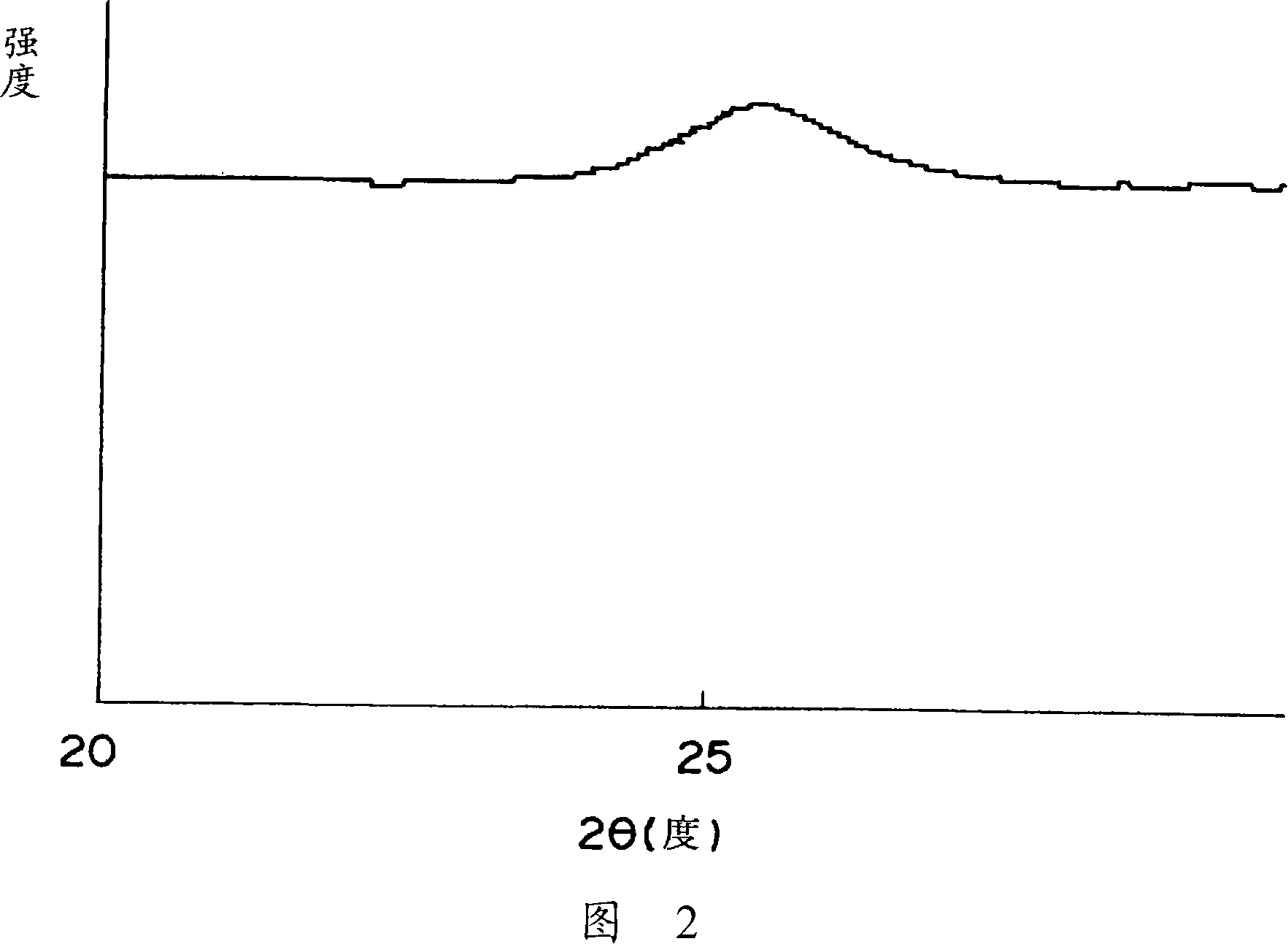
[0062] With 7.1kg silica sol (containing 20 mass % SiO 2) and 140kg ammonia water (containing 10% by mass NH 3 ) mixed, slowly add 60 liters of sulfuric acid aqueous solution of titanyl sulfate (containing 125g / liter TiO 2 , 0.55kg / liter H 2 SO 4 ), forming a precipitate. After the slurry was aged, filtered and washed, it was dried at 150° C. for 10 hours. The product was calcined at 500° C. for 6 hours, and pulverized using a hammer mill to obtain powder A. The average particle size of this powder A was 16 μm. TiO is not visible in the X-ray diffraction pattern of Powder A 2 , SiO 2 It was confirmed from the broad diffraction peak that it was a composite oxide of titanium and silicon (Ti-Si composite oxide) having an amorphous fine structure (see FIG. 1 ).
[0063] 2 kg of the above-mentioned powder A were added and mixed with starch and water as forming aids, and kneaded in a kneader. Water was further added to the obtained kneaded product, and it was mad...
Embodiment 2
[0075]
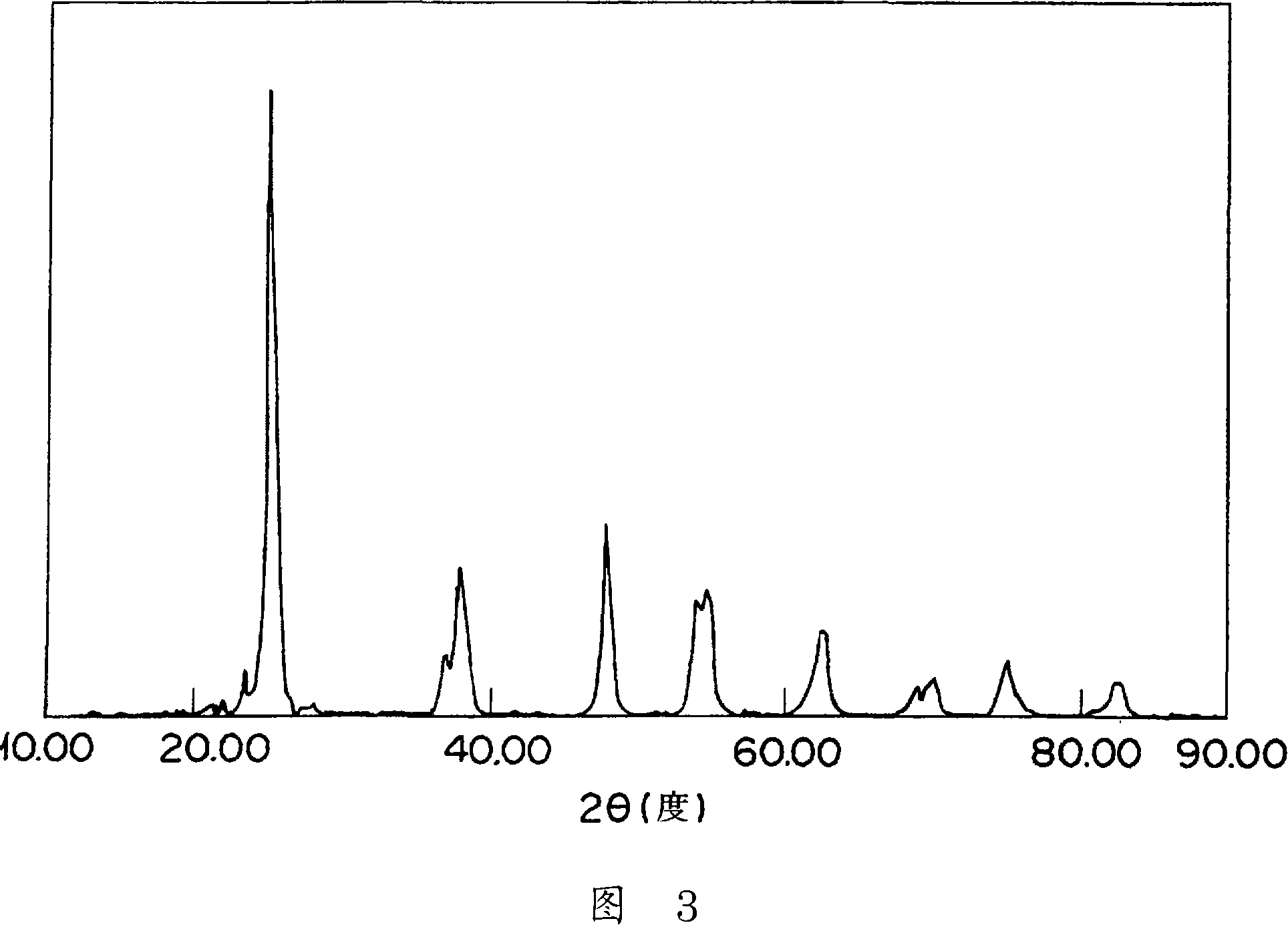
[0076] With 12kg ammonium metatungstate aqueous solution (containing 50 mass % WO 3 ), 400kg ammonia water (containing 25 mass% NH 3 ) mixed with 8kg water, slowly add 779 liters of sulfuric acid aqueous solution of titanyl sulfate (containing 70g / liter TiO 2 , 310g / liter H 2 SO 4 ), forming a precipitate. This slurry was aged, filtered, washed, and dried at 150° C. for 10 hours. The product was calcined at 600°C for 3 hours, and then pulverized with a hammer mill to obtain titanium-tungsten mixed oxide powder B (average particle size 19 μm, Ti-W mixed oxide, TiO 2 : WO 3 =90:10 (mass ratio)). In the X-ray diffraction pattern of this powder B, TiO was confirmed at 2θ=23.5° and 2θ=22°, respectively. 2 and WO 3 The obvious intrinsic peak (refer to Figure 2).
[0077] In the mixture of 18 kg of the above-mentioned powder B and 2 kg of the powder A obtained in Example 1, starch and water as forming aids were mixed, kneaded in a kneader, and then formed into an ...
Embodiment 3
[0087]
[0088] 2 kg of the powder B obtained in Example 2 was added and mixed with starch and water as forming aids, and kneaded in a kneader. Water was further added to the obtained kneaded product, and it was made into slurry in a mixer, and the prepared honeycomb cordierite carrier (outer shape 150 mm square, length 50 mm, mesh 1.4 mm, wall thickness 0.4 mm) was impregnated. Thereafter, the carrier was pulled out, dried at 80°C, and fired at 550°C for 3 hours. The supporting ratio of the Ti-W mixed oxide at this time was 8% by mass.
[0089] This support was immersed in a solution of rhodium nitrate aqueous solution and dinitrodiammine platinum aqueous solution, dried at 150° C. for 3 hours, and calcined at 500° C. for 2 hours in an air atmosphere to obtain catalyst C. The composition of catalyst C is (Ti-W mixed oxide):Rh:Pt=98.5:0.5:1 (mass ratio), and the BET specific surface area of the cordierite part is 82m 2 / g. In addition, the average particle size of plati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com