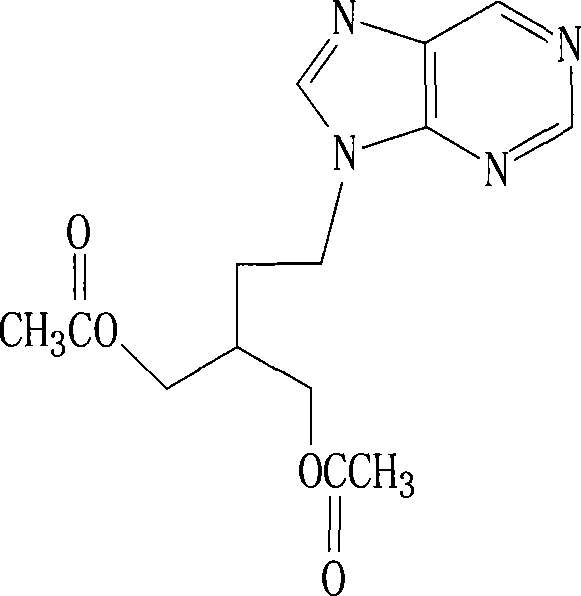
Falacyclovir dispersion piece and its preparation method
A technology of famciclovir and dispersible tablets, which is applied in the field of preparation of famciclovir dispersible tablets, can solve the problems of only swallowing, large single dose, inconvenience, etc., and achieve the effects of convenient taking, low production cost and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Famciclovir 250g
[0027] Microcrystalline Cellulose 200g
[0028] Sodium carboxymethyl starch 40g
[0029] Low-substituted hydroxypropyl cellulose 35g
[0030] Croscarmellose sodium 65g (additional 30g)
[0034] Get the prescription amount of famciclovir, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, croscarmellose sodium, saccharin sodium, sieve, mix evenly, and add 60% polyvinylpyrrolidone (K30) with an appropriate amount of 0.05g / ml concentration % ethanol solution to make soft material, pass through 18 mesh sieve to granulate, dry at 60°C for 4 hours, granulate with 18 mesh sieve, add talcum powder, magnesium stearate, and the remaining croscarmellose sodium, mix evenly, and press into tablets. have to.
Embodiment 2
[0036] Famciclovir 250g
[0037] Pregelatinized starch 176.8g
[0038] Sodium carboxymethyl starch 30g
[0039] Low-substituted hydroxypropyl cellulose 35g
[0040] Cross-linked polyvinylpyrrolidone 45g (plus 20g)
[0041] Aspartame 2.2g
[0042] Talc powder 5.5g
[0043] Magnesium Stearate 5.5g
[0044] Take the prescribed amount of famciclovir, pregelatinized starch, sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose, cross-linked polyvinylpyrrolidone, and aspartame, sieve, mix well, and use an appropriate amount of polyethylene glycol with a concentration of 0.05g / ml The soft material made of pyrrolidone (K30) 60% alcohol solution, granulated through 18 mesh sieve, dried at 60°C for 4 hours, granulated with 18 mesh sieve, added talcum powder, magnesium stearate, and the remaining cross-linked polyvinylpyrrolidone and mixed evenly , ready to be pressed into tablets.
Embodiment 3
[0046] Famciclovir 250g
[0047] Lactose 147g
[0048] Sodium carboxymethyl starch 15g
[0049] Low-substituted hydroxypropyl cellulose 35g
[0050] Cross-linked polyvinylpyrrolidone 40g (plus 25g)
[0051] Aspartame 1.5g
[0052] Micronized silica gel 10g
[0053] Magnesium Stearate 1.5g
[0054] Take the famciclovir, lactose, low-substituted hydroxypropyl cellulose, cross-linked polyvinylpyrrolidone, and aspartame of the prescribed amount, sieve, mix well, and use an appropriate amount of 0.05g / ml concentration of polyvinylpyrrolidone (K30) 60% alcohol solution Make soft material, pass through 18-mesh sieve to granulate, dry at 60°C for 4 hours, granulate with 18-mesh sieve, add talc powder, magnesium stearate, and additional cross-linked polyvinylpyrrolidone, mix evenly, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com