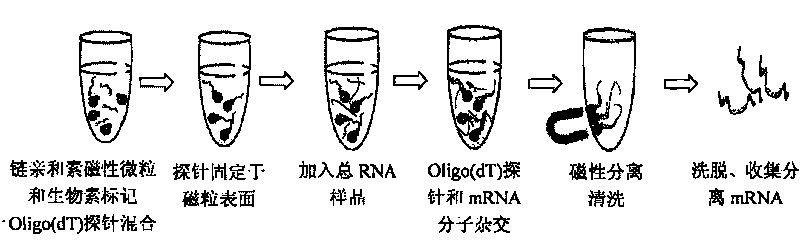
Method for separating mRNA by using gold magnetism particles
A technology of gold magnetic particles and magnetic particles, which is applied in the field of separating mRNA by using gold magnetic particles, can solve the problems of high price, methods that do not involve mRNA separation, unfavorable general use in general laboratories, etc., and achieves reduction of separation costs and immobilization operations Time saving, high fixed capacity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] 1) Preparation of streptavidin-gold magnetic particles;
[0063] 1.1) Take 1mg of gold magnetic particles, equilibrate twice with 0.01MTris-HCl (pH8.0), add 200μg streptavidin, the reaction volume is 500μl, react in a shaker at 37°C, 180r / min for 20min, and react After completion, wash with 2×STE (pH8.0) buffer three times;
[0064] 1.2) Measure the OD value of the supernatant at 280nm before and after streptavidin immobilization and after washing with an ultraviolet-visible spectrophotometer, calculate the coupling efficiency and immobilization amount of streptavidin on the surface of gold magnetic particles, add 1ml containing 0.01%NaN 3 , 2×STE (pH8.0) buffer solution of 1% BSA, stored at 4°C for future use;
[0065] 2) Mix the biotin-labeled oligo(dT) probe with streptavidin-gold magnetic particles, and immobilize the biotinylated oligo(dT) probe on the surface of the magnetic particles through the combination of streptavidin-biotin , as a solid phase carrier for...
Embodiment 1
[0078] This example is the process of immobilizing streptavidin on the surface of gold magnetic particles in the present invention.
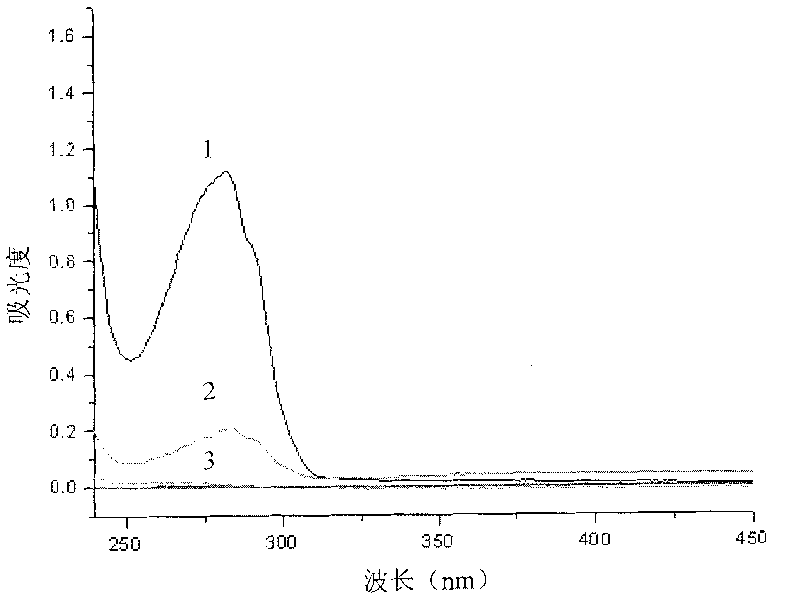
[0079] Take 1 mg of gold magnetic particles, equilibrate twice with 0.01M Tris-HCl (pH 8.0), add 200 μg streptavidin, and the reaction volume is 500 μl. React in a shaker at 37° C. and 180 r / min for 10 min. After the reaction is complete, wash with 2×STE (pH 8.0) buffer three times, 500 μl each time. Ultraviolet-visible spectrophotometer was used to measure the OD value of the supernatant at 280nm before and after streptavidin was immobilized and after washing, and the coupling efficiency and immobilized amount of streptavidin on the surface of gold magnetic particles were calculated. Add 1ml containing 0.01% NaN 3 , 1% BSA in 2×STE (pH 8.0) buffer, stored at 4°C for later use.
[0080] The UV absorption curves before and after the coupling of gold magnetic particles to streptavidin are as follows: figure 2 As shown, wherein curve 1 is the u...
Embodiment 2
[0082] This example is the process of immobilizing biotin-labeled oligonucleotide probes on the surface of streptavidin-gold magnetic particles in the present invention.
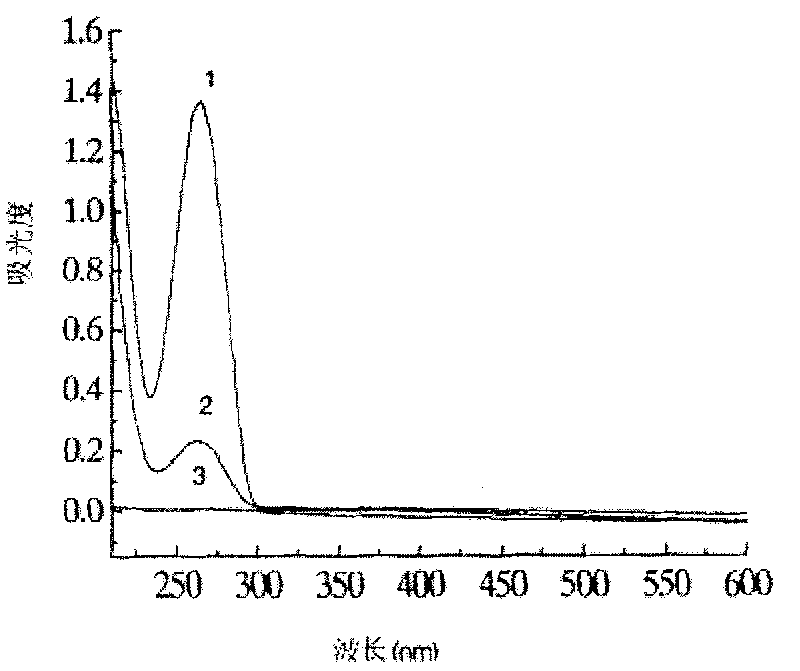
[0083] Take 250 μg streptavidin-gold magnetic particles, equilibrate three times with 2×STE buffer, magnetically separate, add 150 μl biotin-labeled oligonucleotide (dissolved in 2×STE, containing 862.5 pmol oligonucleotide), React in a shaker at 37°C and 120r / min for 10min. After the reaction, magnetically separate and wash thoroughly with 2×STE buffer. Measure the OD value of the supernatant before and after immobilization and after washing at 260nm, and calculate the biological Coupling efficiency and immobilization amount of prime-labeled oligonucleotides on the surface of gold magnetic particles.
[0084] The UV absorption curves before and after immobilization of biotin-labeled oligonucleotide probes on streptavidin-gold magnetic particles are as follows: image 3 As shown, curve 1 is the ultraviolet ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com