Method for producing thermochromism variable emissivity lanthanum manganic acid material
A technology of thermochromism and emissivity, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, etc., can solve the problems of high production cost, not at room temperature, and unsatisfactory, and achieve convenient emissivity, convenient measurement, and convenient mechanical processing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
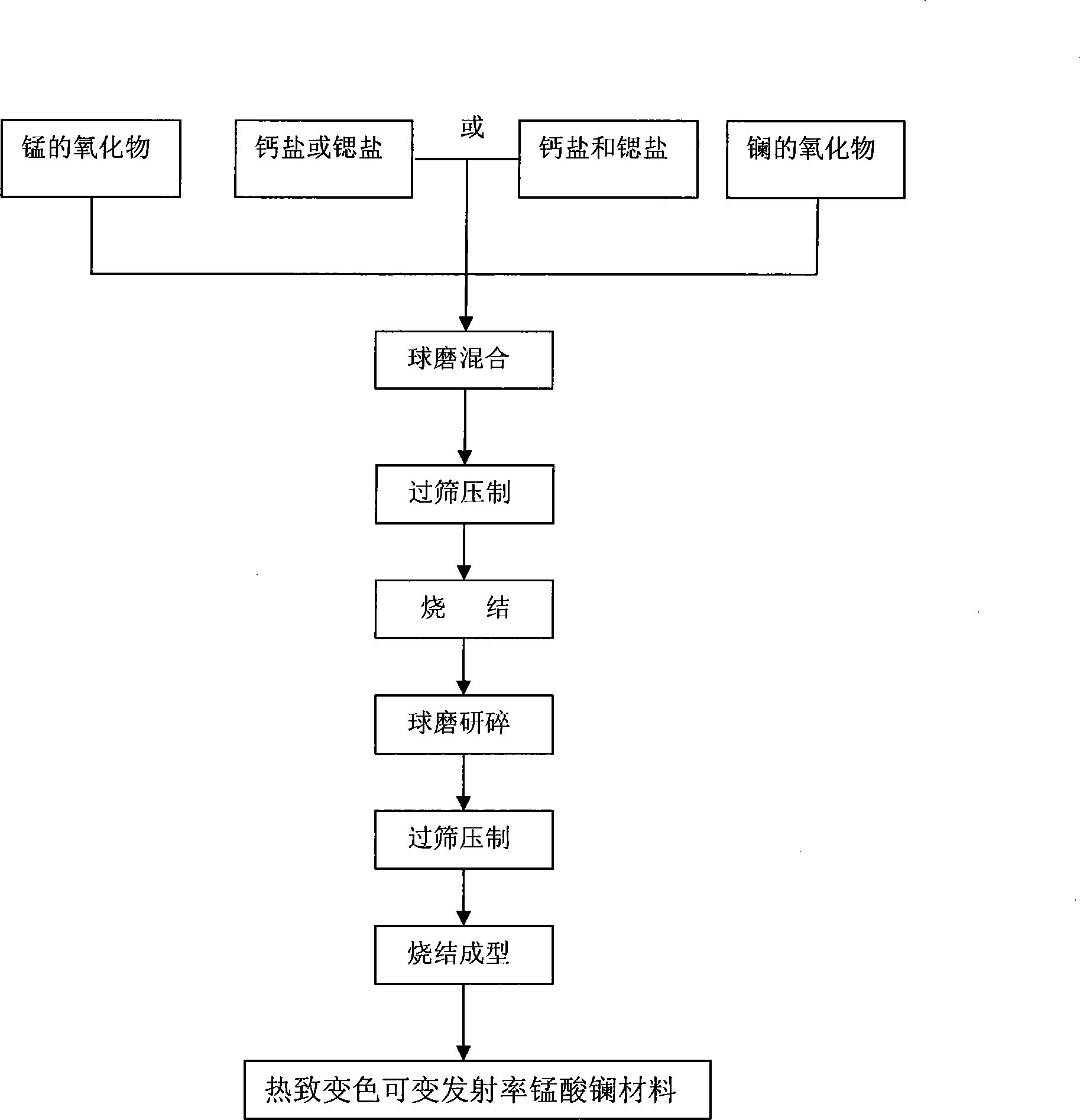
[0016] La 0.75 Ca 0.1 Sr 0.15 MnO 3 Preparation of bulk samples
[0017] 1. According to the molar ratio of metal substances in the molecular formula, accurately weigh La 2 o 3 36.6330 g, SrCO 3 6.6394 g, CaCO 3 3.0153 grams and MnO2 6.0702 grams. Since lanthanum oxide is easy to deliquesce in air, burn it at 1000°C for 4 hours to remove CO 2 and water of crystallization;
[0018] 2. Pour all of it into a ball mill and ball mill at a speed of 300-500r / min for 2-4 hours;
[0019] 3. Pass the mixed powder through a 100-mesh sieve, and then press it into a block under a static pressure of 180Mpa;
[0020] 4. Put the block sample into a high-temperature furnace, and pre-sinter as follows: the initial temperature rises to 1000°C-1200°C after 2-4 hours, and is kept at this temperature for 6-10 hours; finally, the temperature is raised to 1350°C Cool naturally after 15-20 hours of heat preservation to obtain black powder;
[0021] 5. Repeat the second and third steps;
...
Embodiment 2
[0024] La 0.825 Sr 0.175 MnO 3 Preparation of bulk samples
[0025] 1. According to the molar ratio of metal substances in the molecular formula, accurately weigh La 2 o 3 26.0169 g, SrCO 3 5.002 g and MnO 2 16.7290 grams. Since lanthanum oxide is easy to deliquesce in the air, burn it at 1000°C for 4 hours to remove CO 2 and water of crystallization;
[0026] 2. Pour all of it into a ball mill and ball mill at a speed of 300-500r / min for 2-4 hours;
[0027] 3. Pass the mixed powder through a 50-mesh sieve, and then press it into a block under a static pressure of 150Mpa;
[0028] 4. Put the block sample into a high-temperature furnace, and pre-sinter as follows: the initial temperature rises to 1000°C-1200°C after 2-4 hours, and is kept at this temperature for 6-10 hours; finally, the temperature is raised to 1450°C Cool naturally after 4-8 hours of heat preservation to obtain black powder;
[0029] 5. Repeat the second and third steps;
[0030] 6. Put the pressed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com