System for slowing and controlling to release gas bag floationg
A technology of airbags and airbag shells, which can be applied to medical preparations with inactive ingredients, inactive ingredients of oil/fat/wax, pharmaceutical formulations, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] An insoluble air sac shell
[0031] Weigh 30g of cellulose acetate and dissolve it in 500ml of acetone, then keep stirring until completely dissolved. Then dissolve 2g of PEG6000 in 30ml of pure water, and keep stirring until completely dissolved. Mix the above two solutions and stir well. Let it stand until the air bubbles in the solution are removed. Put the cured glue solution into the glue dipping tank. The temperature of the liquid in the glue dipping tank is 40°C, and the indoor environment temperature is 20-25°C. The capsule shell template is automatically glued, dried, shelled, and cut. That is, an insoluble air bag shell was obtained. Airbag shells with different inner diameters can be obtained according to different capsule shell templates.
Embodiment 2
[0033] Famotidine double-chamber single-layer balloon floating osmotic pump
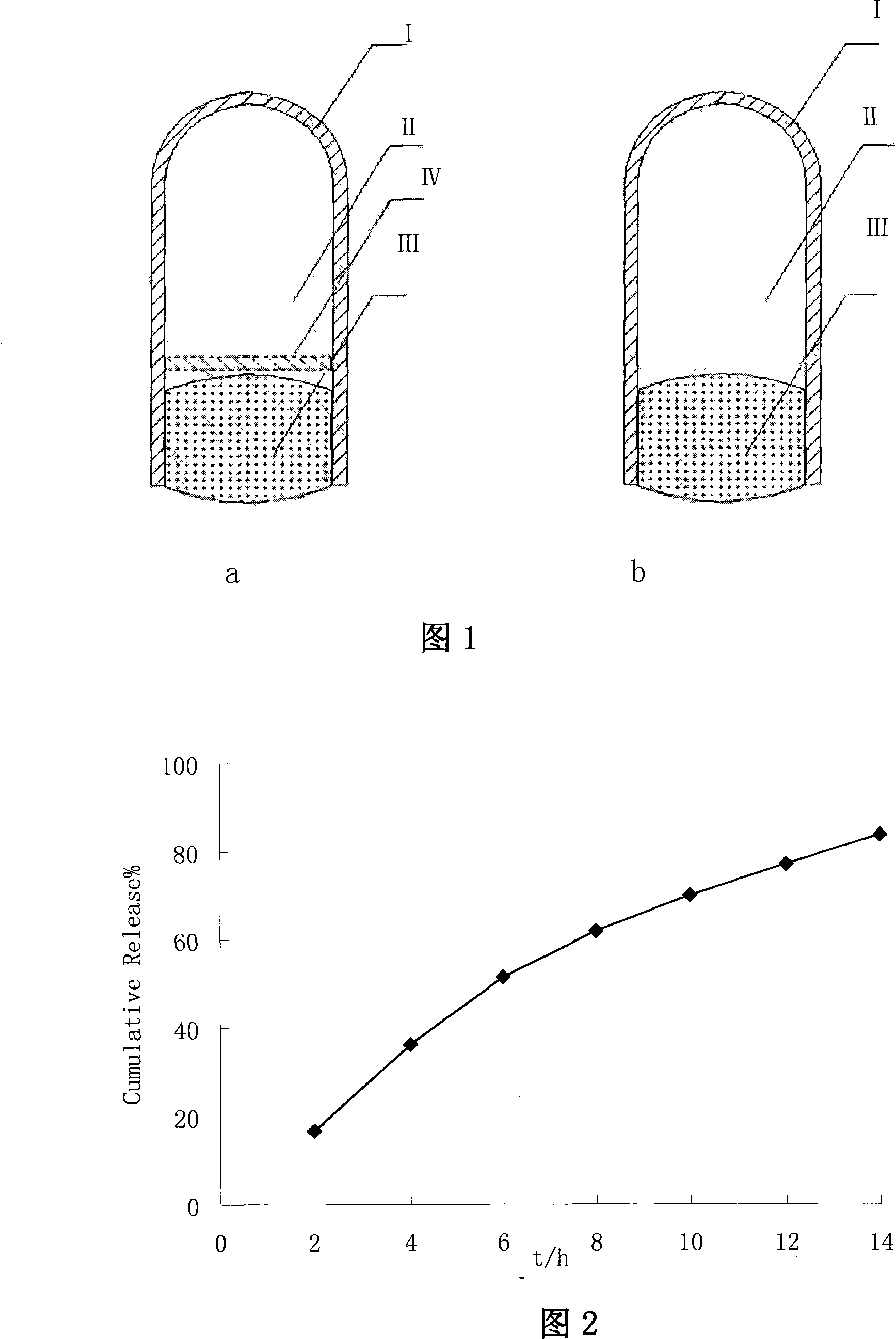
[0034] In Example 1 as shown in Figure 1, the airbag shell I is a No. 0 enteric-coated capsule cap; the sustained and controlled release preparation III containing the drug is a famotidine single-layer osmotic pump tablet.
[0035] Famotidine Monolayer Osmotic Pump Tablets:
[0036] Tablet Prescription:
[0037] Famotidine 13.3%
[0038] Glucose 46.7%
[0039] Tragacanth 33.3%
[0040] Microcrystalline Cellulose 13.3%
[0041] 5% PVP ethanol solution appropriate amount
[0042] Magnesium Stearate Appropriate amount
[0043] 1000 pieces
[0044] Coating solution:
[0045] Cellulose acetate 30g
[0046] PEG4000 24g
[0047] Dibutyl phthalate 4g
[0048] Acetone 1000ml
[0049] water 100ml
[0050] Raw and auxiliary materials such as famotidine, glucose, tragacanth gum, microcrystalline cellulose, polyvinylpyrrolidone (PVPK90), magnesium stearate, etc. are pulverized until ...
Embodiment 3
[0056] Famotidine Gel Skeletal Airbag Floating Preparation
[0057] In Example 1 as shown in Figure 1, the airbag shell I is a No. 0 enteric-coated capsule; the sustained and controlled release preparation III containing the drug is a famotidine gel matrix tablet.
[0058] Famotidine Gel Matrix Tablets:
[0059] Tablet Prescription:
[0060] Famotidine 26.7%
[0061] Polyoxyethylene (molecular weight: 300,000) 33.3%
[0063] 5% PVP ethanol solution appropriate amount
[0064] Magnesium Stearate Appropriate amount
[0065] 1000 pieces
[0066] Raw and auxiliary materials such as famotidine, polyoxyethylene, sodium chloride, polyvinylpyrrolidone (PVPK30), magnesium stearate, etc. were pulverized until passing through an 80-mesh sieve. Weigh 40 g of famotidine, 10 g of sodium chloride, and 90 g of polyoxyethylene, and mix them uniformly. Use 5% PVP ethanol solution as a binder to make soft materials, pass through a 18-m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com