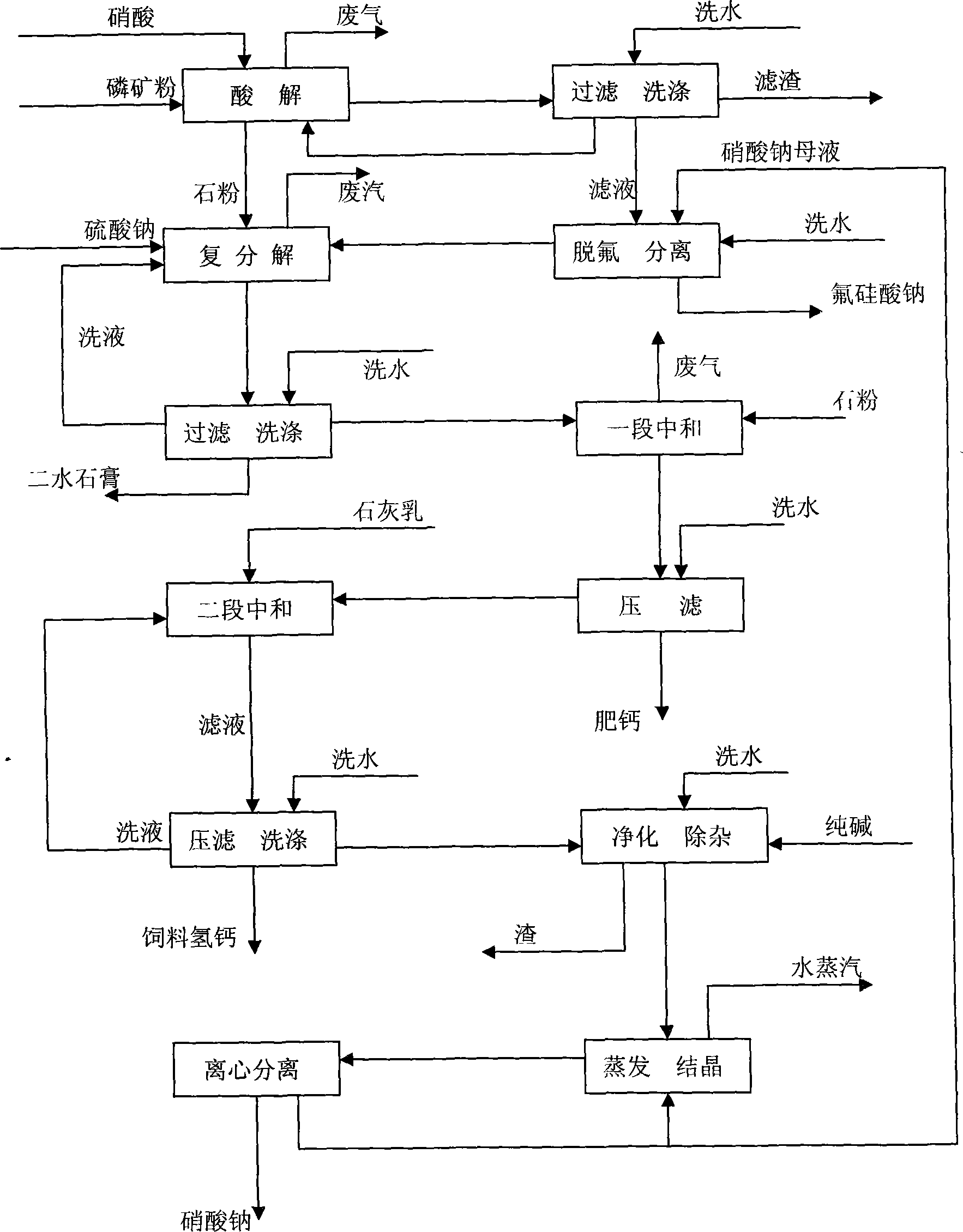
Method for producing calcium hydrophosphate and sodium nitrate by decomposing phosphorus ore with nitric acid
A technology of calcium hydrogen phosphate and sodium nitrate, applied in chemical instruments and methods, preparation of calcium carbonate/strontium/barium, alkali metal nitrate, etc., can solve problems such as unsuitable process and consumption of sulfur resources, etc., and achieve easy treatment and discharge The effect of less waste, good economic benefits and environmental benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0041] Embodiment: adopt the process of decomposing phosphate rock with nitric acid of the present invention, decalcifying Glauber's salt, and neutralizing with stone powder and milk of lime to produce feed calcium hydrogen phosphate and co-produce sodium nitrate:
[0042] The first step acid hydrolysis
[0043] Mix nitric acid with a mass fraction of 50% and filter residue washing solution, add it to the acidolysis tank, and add phosphate rock powder produced in Weng'an, Guizhou under stirring for acidolysis. The acidolysis temperature is 50°C-60°C, and the residence time is 2-4h (The amount of nitric acid is 90% to 100% of the theoretical acid consumption); after the reaction, the acid solution is filtered, and the filter residue is washed with clear water; the washing solution is returned and mixed with nitric acid to acidify the phosphate rock powder.
[0044] The fineness of phosphate rock powder produced in Weng'an is 62.3% over 100 mesh and 65.0% over 80 mesh. Its composi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com