Liquid crystal monomer with reaction character and preparation method thereof
A technology of liquid crystal monomers and characteristics, applied in the field of liquid crystal compounds in the chemical field, can solve the problems of unstable product performance and easy adsorption of other ionic impurities, and achieve the effect of broadening the scope of application and increasing stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
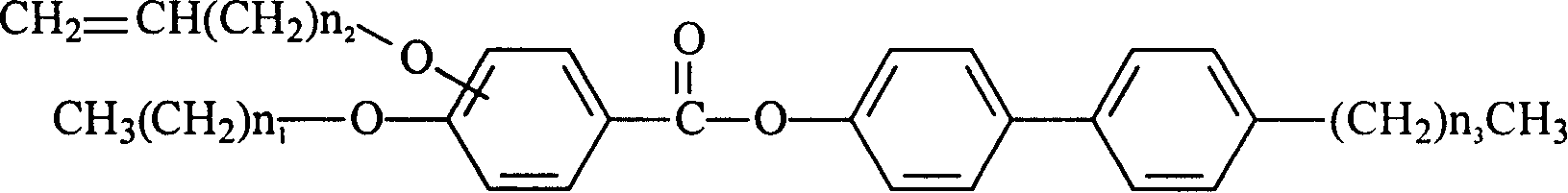
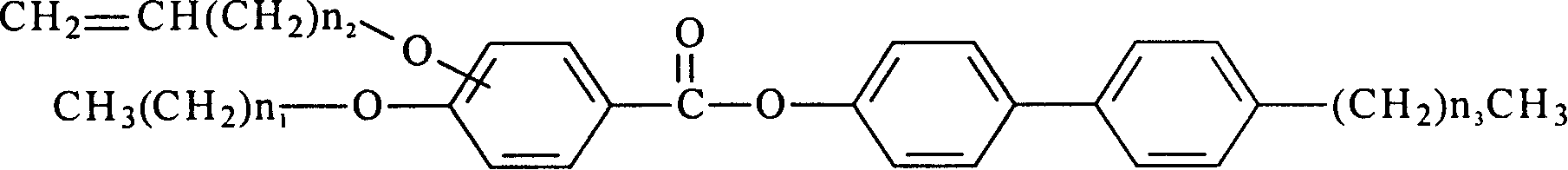
[0018] The structural formula of the liquid crystal monomer with reactive properties in this embodiment is:
[0019]
[0020] where: n 1 =7,n 2 = 1, n 3 =2, alkenyloxy ortho-substituted.
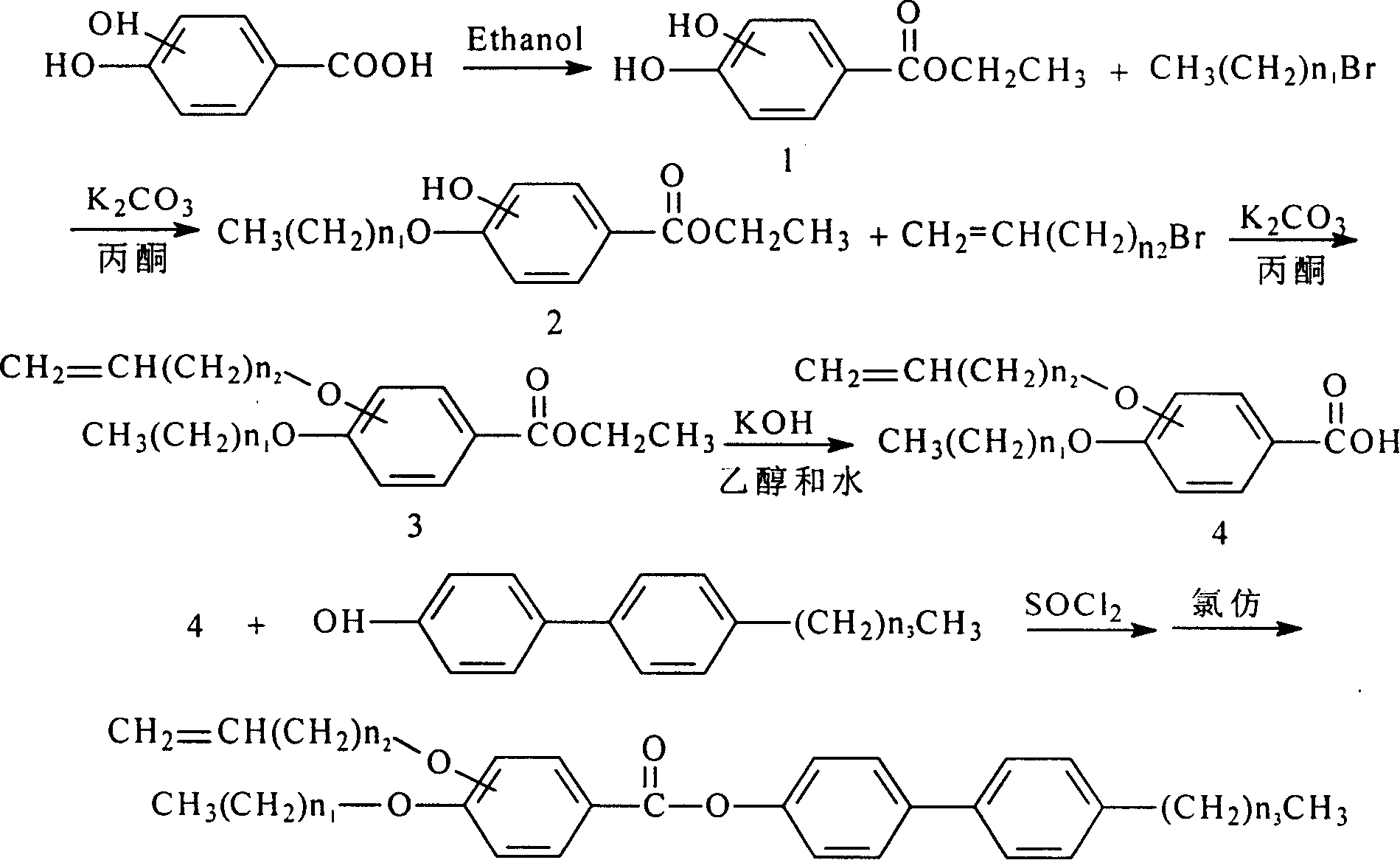
[0021] The synthetic method of liquid crystal monomer 2-allyloxy-4-octyloxybenzoic acid p-propyl biphenyl ester:
[0022] (1) Ethyl 2,4-dihydroxybenzoate: Weigh 8g of 2,4-dihydroxybenzoic acid into a 250ml three-necked round-bottomed flask, then add 40ml of ethanol, heat it up to 40°C, and add 3ml of concentrated sulfuric acid dropwise , 30min to complete, heating to reflux. Stop the reaction after 10 hours, cool to room temperature, add anhydrous potassium carbonate to the three-necked flask to adjust the pH of the solution to 7, filter with water pump, wash the filter residue with 5ml of ethanol, then concentrate the filtrate, recover 35ml of solvent, and cool to obtain 9.99g of a reddish crude product , recrystallized with water, decolorized with activated carbon, and finally obta...
Embodiment 2
[0028] The structural formula of the liquid crystal monomer with reactive properties in this embodiment is:
[0029]
[0030] where: n 1 =9,n 2 = 3, n 3 =2, alkenyloxy ortho-substituted.
[0031] The synthetic method of liquid crystal monomer 2-pentyloxy-4-decyloxybenzoic acid p-propyl biphenyl ester:
[0032] (1) Ethyl 2,4-dihydroxybenzoate: Weigh 8g of 2,4-dihydroxybenzoic acid into a 250ml three-necked round-bottomed flask, then add 40ml of ethanol, heat it up to 40°C, and add 3ml of concentrated sulfuric acid dropwise , 30min to complete, heating to reflux. Stop the reaction after 10 hours, cool to room temperature, add anhydrous potassium carbonate to the three-necked flask to adjust the pH of the solution to 7, filter with water pump, wash the filter residue with 5ml of ethanol, then concentrate the filtrate, recover 35ml of solvent, and cool to obtain 9.99g of a reddish crude product , recrystallized with water, decolorized with activated carbon, and finally obt...
Embodiment 3
[0038] The structural formula of the liquid crystal monomer with reactive properties in this embodiment is:
[0039]
[0040] where: n 1 =7,n 2 = 1, n 3 =3, alkenyloxy meta-substituted.
[0041] The synthetic method of liquid crystal monomer 3-allyloxy-4-octyloxybenzoic acid p-butyl biphenyl ester:
[0042] (1) Ethyl 3,4-dihydroxybenzoate: Weigh 8g of 3,4-dihydroxybenzoic acid into a 250ml three-necked round-bottomed flask, then add 40ml of ethanol, heat it up to 40°C, and add 3ml of concentrated sulfuric acid dropwise , 30min to complete, heating to reflux. Stop the reaction after 10 hours, cool to room temperature, add anhydrous potassium carbonate to the three-necked flask to adjust the pH of the solution to 7, filter with water pump, wash the filter residue with 5ml of ethanol, then concentrate the filtrate, recover 35ml of solvent, and cool to obtain 9.9g of a reddish crude product , recrystallized with water, decolorized with activated carbon, and finally obtaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com