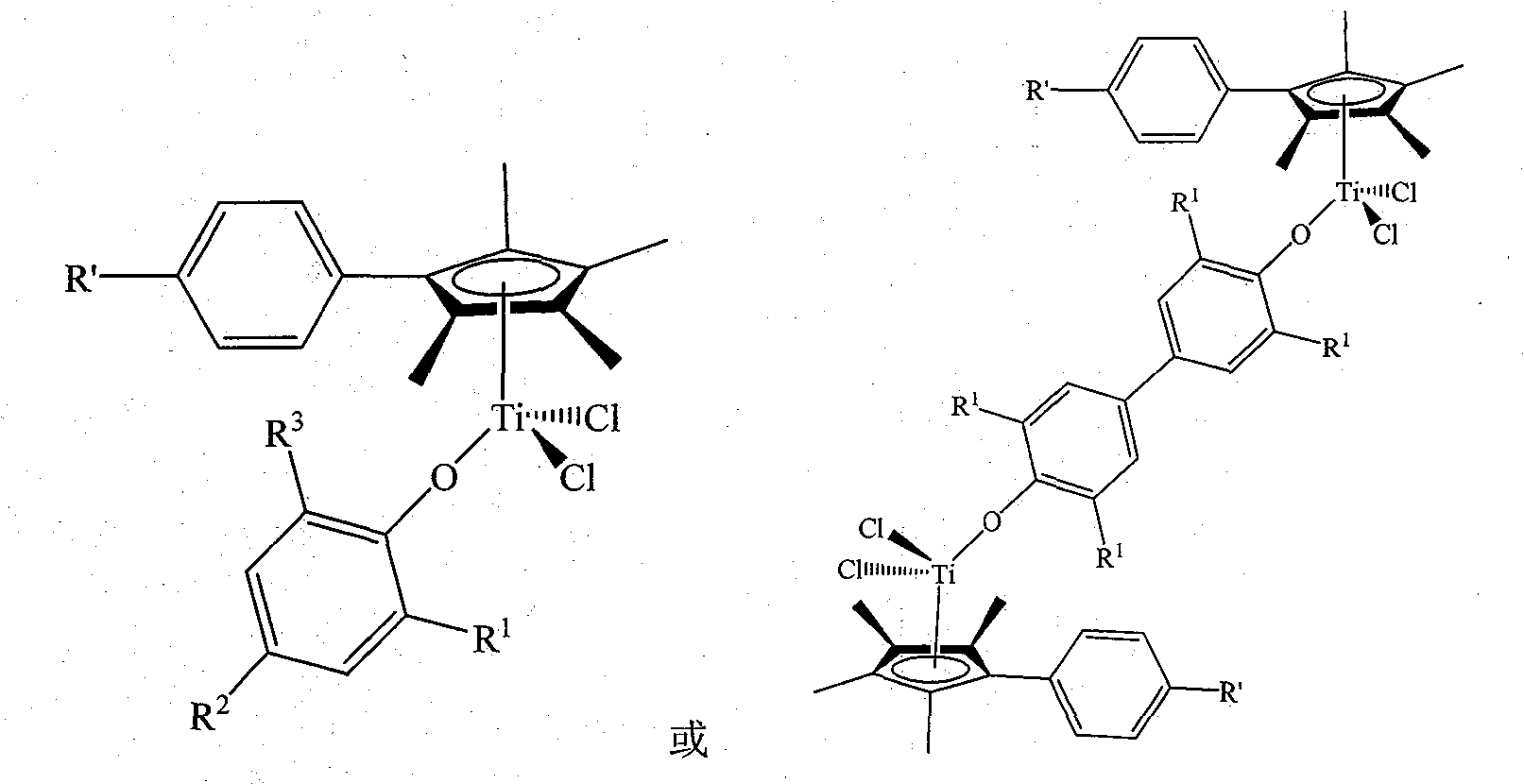
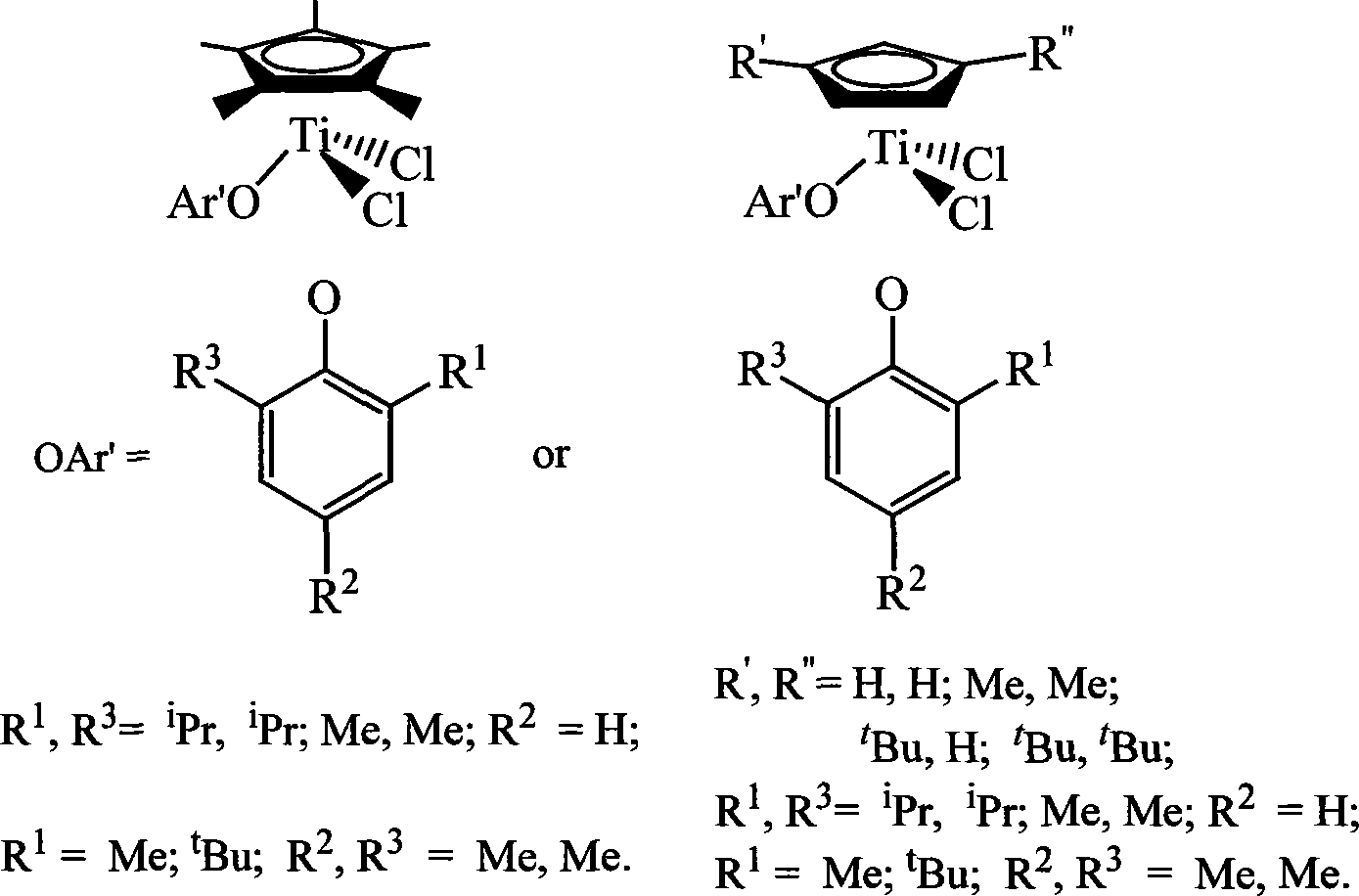
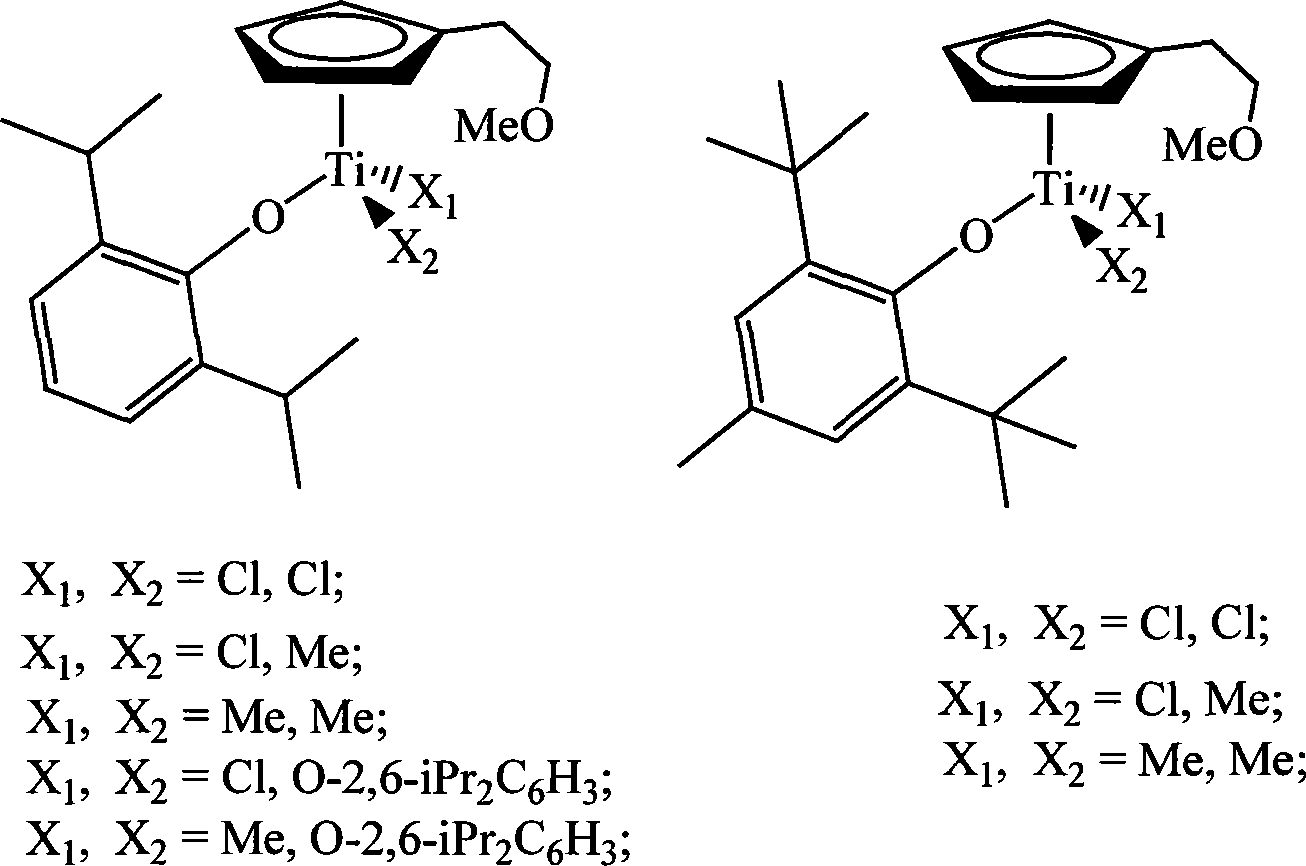
Non-bridged single/double-nucleus metallocene compound and uses thereof
A metallocene compound and bridging technology, which is applied to olefin polymerization catalysts and their preparation and application fields, can solve problems such as unfavorable catalyst stability, reduced catalytic activity and the like, and achieve the effect of improving application scope and added value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Synthesis of 1-(4-methyl)-phenyl-2,3,4,5-tetramethylcyclopentadienyl ligand The reaction equation of the synthesis is:
[0037]
[0038] The specific process is: in N 2 Under the atmosphere, add 15.9ml 2,3,4,5-tetramethylcyclopent-2-en-1-one (36mmol) ether solution to the reaction flask, slowly drop 18ml, 2.0 under ice salt conditions M 4-methyl-phenyl lithium salt (4-Me-PhLi, 36 mmol) in ether. Under stirring conditions, gradually rise to room temperature and react for about 30 hours. The reaction mixture was poured into cold water, and the pH was adjusted to about 1 with hydrochloric acid. The aqueous layer was extracted with ether, and the combined organic phase was washed with 120 ml of saturated ammonium chloride aqueous solution, and then MgSO 4 dry. The desiccant was removed by filtration, the solvent was evaporated under reduced pressure, and column chromatography was separated (developing solvent: dichloromethane / petroleum ether = 1:1 mixed solvent) to...
Embodiment 2
[0041] Example 2 Synthesis of 1-(4-isopropyl)-phenyl-2,3,4,5-tetramethylcyclopentadienyl ligand
[0042] Under nitrogen atmosphere, add 15.90ml of ether solution containing 2,3,4,5-tetramethylcyclopent-2-en-1-one (36mmol) into the reaction flask, and slowly Add 20ml, 1.8M 4-isopropyl-phenyllithium salt (4-ipr-PhLi, 36mmol) in ether. . Under stirring conditions, gradually rise to room temperature and react for 30 hours. The reaction mixture was poured into ice water, and the pH was adjusted to about 1 with hydrochloric acid. The aqueous layer was extracted with ether, and the combined organic phase was washed with a saturated aqueous solution of ammonium chloride (120 ml) and then MgSO 4 dry. The desiccant was removed by filtration, the solvent was evaporated under reduced pressure, and the product was separated by chromatography (developing solvent: dichloromethane / petroleum ether = 1:3 mixed solvent) to obtain 6.66 g of the product, with a yield of 77%.
[0043] 1 Analysis of ...
Embodiment 3
[0045] Example 3 Synthesis of 1-(4-tert-butyl)-phenyl-2,3,4,5-tetramethylcyclopentadienyl ligand
[0046] Under nitrogen atmosphere, add 15.90ml of ether solution containing 2,3,4,5-tetramethylcyclopent-2-en-1-one (36mmol) into the reaction flask, and slowly Add 22.5ml, 1.6M 4-tert-butyl-phenyllithium salt (4-tbu-PhLi, 36mmol) in ether. . Under stirring conditions, gradually rise to room temperature and react for 30 hours. The reaction mixture was poured into ice water, and the pH value was adjusted to about 1 with hydrochloric acid. The aqueous layer was extracted with ether, and the combined organic phases were washed with a saturated aqueous solution of ammonium chloride (120 ml), and then MgSO 4 dry. The desiccant was removed by filtration, the solvent was evaporated under reduced pressure, and the product was separated by chromatography (developing solvent: dichloromethane / petroleum ether = 1:3 mixed solvent) to obtain 7.23 g of the product, with a yield of 79%.
[0047] 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com