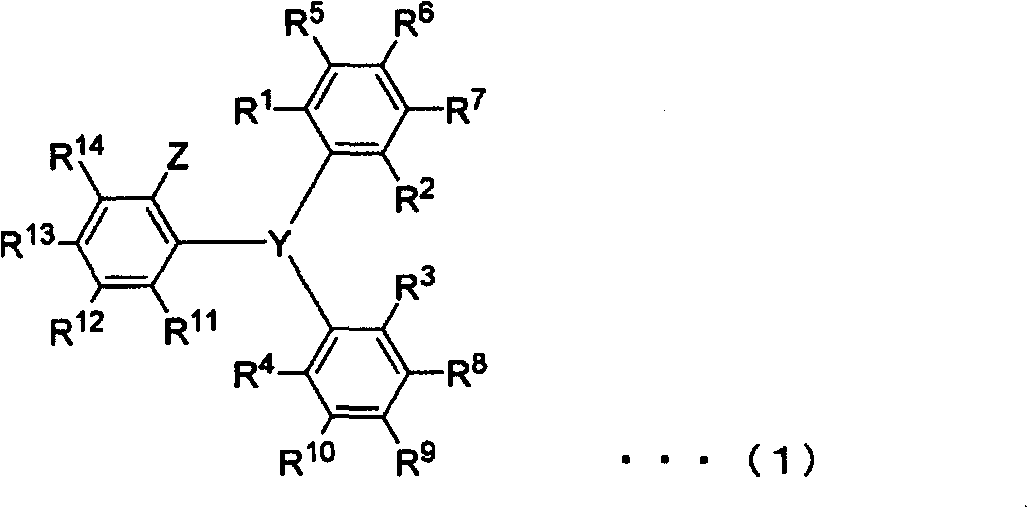
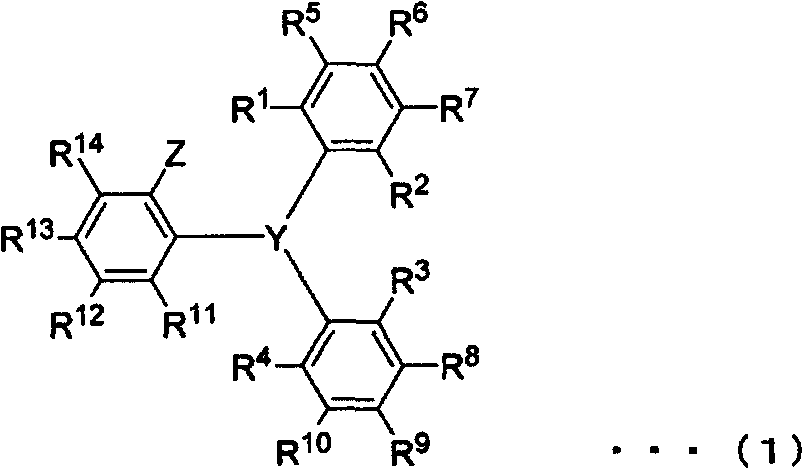
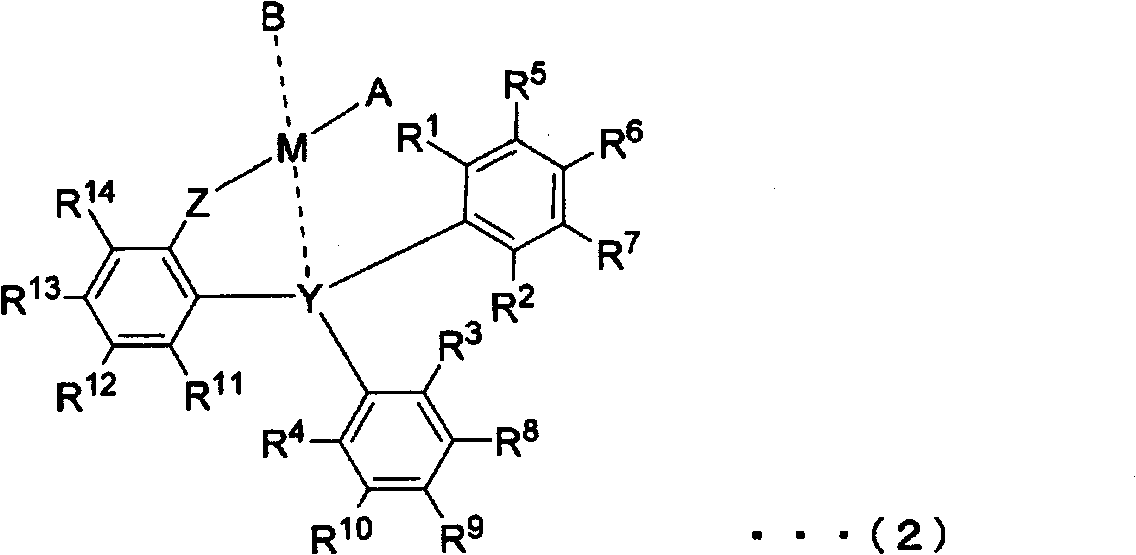
Novel triarylphosphine or triarylarsine compounds, alpha-olefin polymerization catalysts using same, terpolymer, and process for production of copolymer of alpha-olefin and (meth)acrylic comonomer
A technology for olefin polymerization and catalyst, which is applied in nickel organic compounds, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc. It can solve the problem of sticky components and improve the balance between mechanical properties and adhesion Problems such as insufficient mechanical properties of ethylene-based copolymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0151] Examples of the monomer used for the preparation of the copolymer include α-olefins, (meth)acrylic olefins, and other olefins described below.
[0152] (a) α-Olefins
[0153] One of the monomers used in the present invention is the general formula CH 2 =CHR 17 The shown α-olefin (hereinafter may be referred to as "(a) component"). Here, R 17 is hydrogen or an alkyl group having 1 to 20 carbon atoms.
[0154] Among them, R having 1 to 10 carbon atoms is exemplified as a preferable component (a). 17 of α-olefins. As more preferable (a) component, ethylene, propylene, 1-butene, 1-pentene, 1-hexene, 1-octene, 1-decene, 3-methyl-1-butene are mentioned , 4-methyl-1-pentene, more preferably propylene, 1-butene, 1-pentene, 1-hexene, 1-octene, particularly preferably 1-hexene. Moreover, (a) component may be used individually, and multiple types (a) component may be used in combination.
[0155] (b) (meth)acrylic olefins
[0156] Another monomer used in the present inven...
Embodiment
[0219] The present invention will be specifically described below by the examples and comparative examples, and by the data of each suitable example and the contrast of each example and each comparative example, the rationality and significance of the technical solution of the present invention and the comparison with existing ones are confirmed. There is technical excellence. In addition, the structures of ligands used in Examples and Comparative Examples are shown in Table 1.
[0220] [Table 1]
[0221]
[0222] In addition, the following abbreviations are used in the examples.
[0223] Pd(dba)2: Bis(dibenzylideneacetone)palladium
[0224] Ni(cod)2: bis(cyclooctadiene) nickel
[0225] MA: methyl acrylate
[0226] EA: ethyl acrylate
[0227] tBA: tert-butyl acrylate
[0228] MMA: methyl methacrylate
[0229] AA: Acrylic
[0230] VA: vinyl acetate
[0231] LUA: lauryl acrylate
[0232] HEA: 2-Hydroxyethyl Acrylate
[0233] EUA: ethyl undecylenate
[0234] NBMOH:...
Synthetic example 1
[0317] (Synthesis Example 1) Synthesis of Ligand (I)
[0318] Slowly add n-butyllithium hexane solution (2.5M, 2mL, 5mmol) dropwise to a solution of benzenesulfonic anhydride (400mg, 2.5mmol) in tetrahydrofuran (20mL) at 0°C and stir while raising the temperature to room temperature 1 hour. The reaction solution was cooled to -70°C, phosphorus trichloride (340 mg, 2.5 mmol) was added, and the mixture was stirred for 2 hours while raising the temperature to room temperature (reaction solution A).
[0319] Slowly add n-butyllithium hexane solution (2.5M, 2mL, 5mmol) dropwise into a solution of 1-bromo-2-isopropylbenzene (1g, 5mmol) in diethyl ether (20mL) at -30°C. The mixture was stirred for 3 hours while allowing the temperature to rise to room temperature. This solution was added dropwise to the previous reaction solution A at room temperature, and stirred overnight. After the reaction, water (20 mL) was added, extracted with ether (20 mL×2), washed with 1N hydrochloric ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com