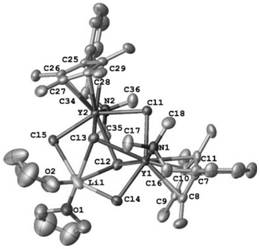
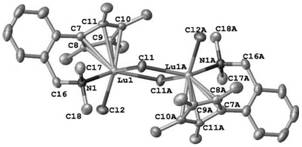
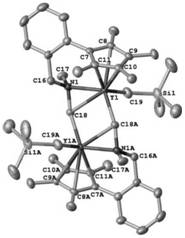
Monocene trivalent transition metal complex containing neutral benzyl heteroatom ligand and use
A transition metal and heteroatom technology, applied in the field of olefin polymerization catalysts, can solve the problems of low polymer molecular weight and comonomer content, wide molecular weight distribution, and low catalytic activity, and achieve high molecular weight, low cost, and high catalytic activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1N, the synthesis of N-dimethyl 1-(2-(2,3,4,5-tetramethylcyclopentadienyl) benzyl)amine titanium dichloride (complex I-1)
[0025] N,N-Dimethylbenzylamine (13.5 g, 0.100 mol) was weighed into a two-neck round bottom flask, and dry diethyl ether (100 mL) was added thereto under nitrogen protection. At room temperature, a hexane solution of n-butyllithium (2.50 mol / L, 40 mL) was added in batches, and after the addition, the reaction solution was stirred and refluxed for 30 h, and then cooled to room temperature. 2,3,4,5-Tetramethyl-2-cyclopentanone (13.8 g, 0.100 mol) was added dropwise to the above reaction system, and the dropping time was over 30 min to keep the reaction under reflux. After the addition, the reaction mixture was stirred and refluxed for 2h. The reaction solution was cooled with an ice-water bath and 6mol / L hydrochloric acid (75mL) was added thereto, and the dark red liquid obtained after removing the volatile matter under reduced pressure w...
Embodiment 2
[0028] Example 2 Synthesis of 1-(2-(2,3,4,5-tetramethylcyclopentadienyl)benzyl)pyrrolidine titanium dichloride (complex I-2)
[0029] Weighed 1-(benzyl)pyrrolidine (16.1 g, 0.100 mol) into a two-neck round bottom flask, and added dry diethyl ether (50 mL) thereto under nitrogen protection. At room temperature, a hexane solution of n-butyllithium (2.50 mol / L, 40 mL) was added in batches, and after the addition, the reaction solution was stirred and refluxed for 30 h, and then cooled to room temperature. 2,3,4,5-Tetramethyl-2-cyclopentanone (13.8 g, 0.100 mol) was added dropwise to the above reaction system, and the dropping time was over 30 min to keep the reaction under reflux. After the addition, the reaction mixture was stirred and refluxed for 2h. The reaction solution was cooled with an ice-water bath and 6mol / L hydrochloric acid (75mL) was added thereto, and the dark red liquid obtained after removing the volatile matter under reduced pressure was redissolved in water (5...
Embodiment 3
[0031] Example 3N, the synthesis of N-dimethyl 1-(2-(9-fluorenyl) benzyl)amine titanium dichloride (complex I-3)
[0032] Weigh N,N-dimethylbenzylamine (13.5 g, 0.100 mol) into a two-neck round bottom flask, and add dry diethyl ether (50 mL) therein under nitrogen protection. At room temperature, a hexane solution of n-butyllithium (2.50 mol / L, 40 mL) was added in batches, and after the addition, the reaction solution was stirred and refluxed for 30 h, and then cooled to room temperature. A solution of 9-fluorenone (18.0 g, 0.100 mol) in diethyl ether (100 mL) was slowly added to the reaction solution over 30 min to keep the reaction under reflux. After the addition, the reaction mixture was stirred and refluxed for 2h. with saturated NH 4 The reaction was quenched with aqueous Cl (30 mL), the organic phase was separated, and the aqueous phase was extracted with ethyl acetate (3 x 30 mL). The combined organic phases were washed with anhydrous MgSO 4 Dry and remove solvent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com