Macromolecule thiolated modified derivatives and cross-linking material thereof
A polymer compound and polymer technology, applied in the field of compounds, can solve the problems of ineffective biomedical applications, poor chemical reaction performance, restricted collision probability, etc., to achieve adjustable performance, high yield, side chain chemistry, etc. Flexible and changeable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1. Synthesis of dithiodipropionic acid bisacylglycine dihydrazide (abbreviated as DGDTPDH)
[0082] Add 10 g of dithiodipropionic acid (Aldrich, USA) and 50 ml of anhydrous dimethylformamide into a 1000 ml beaker. After stirring and dissolving at room temperature, 17.0 g of carbonyldiimidazole (Aldrich, USA) was added. At this time, the solution produced a large number of carbon dioxide bubbles and white precipitates. Reaction under reduced pressure at room temperature for 3 hours. Then 14.7 g of glycine ethyl ester hydrochloride (Aldrich, USA) was added, and the reaction was stirred for 1 hour. Then 500 ml of ether was added, and the mixture was allowed to stand still for 1 hour. Carefully pour off the upper organic phase, then add 100 mL of ethanol and 10 mL of hydrazine hydrate. After stirring overnight at room temperature, the precipitated product was collected by filtration. The precipitate was rinsed twice with 200 ml of absolute ethanol, and then dri...
Embodiment 2
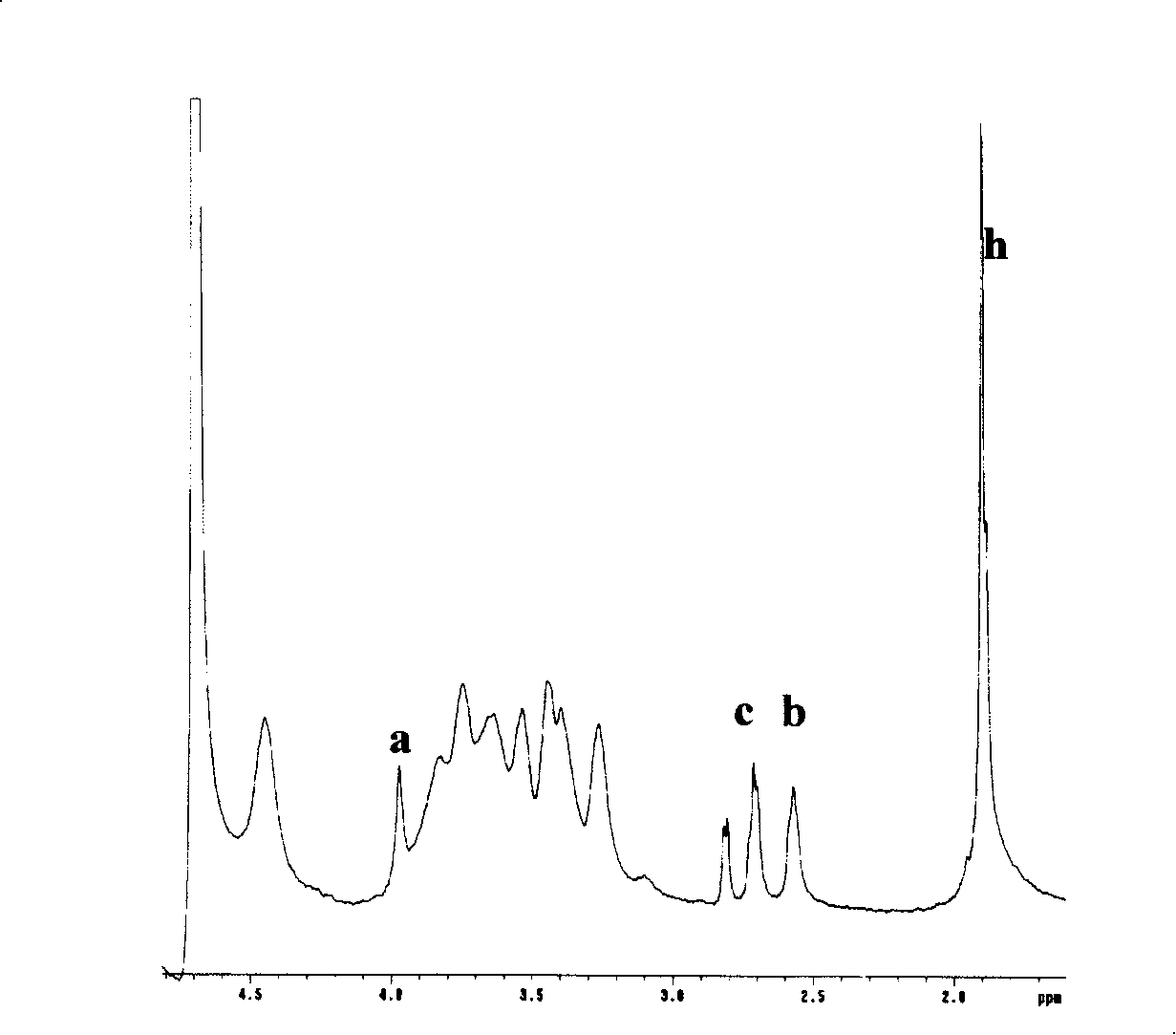
[0083] Example 2. Synthesis of low-substituted DGDTPDH modified hyaluronic acid mercapto derivatives (HA-DGDTPDH)
[0084] Dissolve 1 g of sodium hyaluronate (molecular weight: 620,000 to 1,150,000, NovaMatrix FMC BIOPOLYMER, USA) in 200 ml of distilled water to obtain a clear and transparent solution. Add 1.32 g of DGDTPDH prepared in Example 1 to the above solution, and stir to dissolve. Then the pH value of the solution was adjusted to 4.75 with 0.1 mol / L hydrochloric acid, 0.36 g of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (Aldrich, USA) was added, and electromagnetically stirred. An appropriate amount of 0.1 mol / L hydrochloric acid was continuously added to the above solution to keep the pH value of the solution at 4.75. The viscosity of the solution continued to increase and formed a gel in about 15 minutes. After the gel was formed, the reaction was left at room temperature for 2 hours. Then 10 g of dithiothreitol (Diagnostic Chemical Limited, USA)...
Embodiment 3
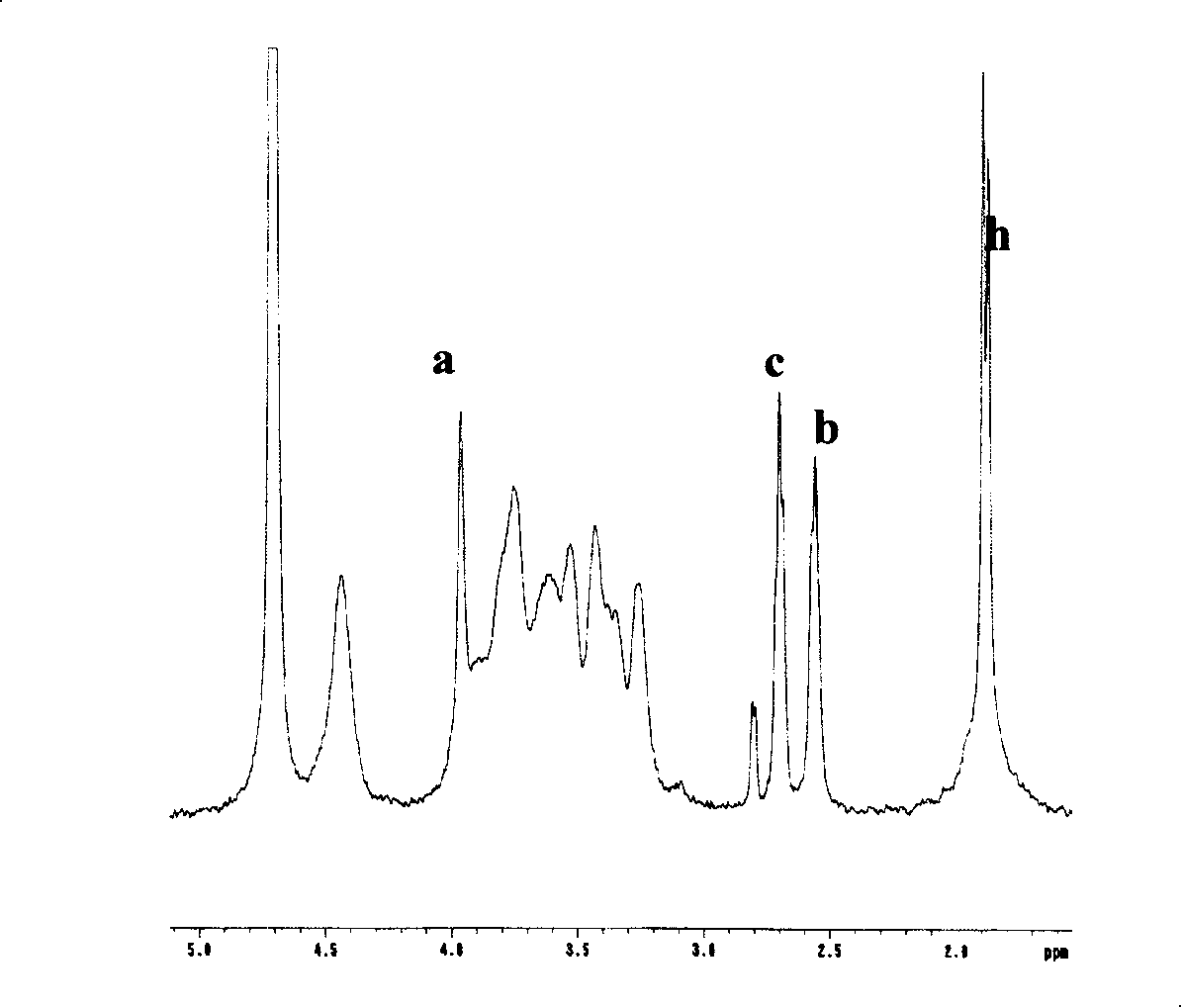
[0085] Example 3. Synthesis of highly substituted DGDTPDH modified hyaluronic acid mercapto derivatives (HA-DGDTPDH)
[0086] Dissolve 1 g of sodium hyaluronate (molecular weight: 620,000 to 1,150,000, NovaMatrix FMC BIOPOLYMER, USA) in 200 ml of distilled water to obtain a clear and transparent solution. Add 2.64 g of DGDTPDH prepared in Example 1 to the above solution, and stir to dissolve. Then the pH value of the solution was adjusted to 4.75 with 0.1 mol / L hydrochloric acid, 0.96 g of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (Aldrich, USA) was added, and electromagnetically stirred. An appropriate amount of 0.1 mol / L hydrochloric acid was continuously added to the above solution to keep the pH value of the solution at 4.75. The viscosity of the solution continued to increase, and a gel formed in about 10 minutes. After the gel was formed, the reaction was left at room temperature for 2 hours. Then 20 g of dithiothreitol (Diagnostic Chemical Limited, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com