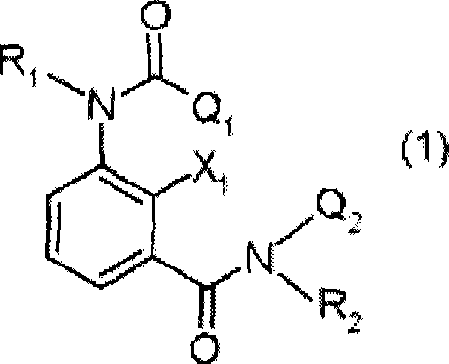
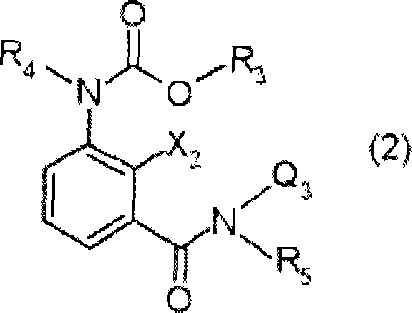
Insecticidal and bactericidal composition
An insecticidal and sterilizing and composition technology, which is applied in the field of new insecticidal and sterilizing compositions, can solve the problem of not being able to control diseases and pests at the same time, and achieve the effects of simultaneously preventing and controlling diseases and pests, saving labor, reducing burden, and being effective.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0259] In all the preparation methods shown above, after the completion of the reaction, the target substance may be separated from the reaction system according to a usual method, and operations such as recrystallization, column chromatography, distillation, etc. may be performed as necessary. In addition, the target substance may be used in the next reaction step without separating it from the reaction system.
[0260] The compound represented by the general formula (2) can be prepared according to the method described in the specification of International Publication No. 2005 / 21488.
[0261] The compound represented by the general formula (3) can be prepared according to the method described in the specification of International Publication No. 2003 / 8372.
[0262] The compound represented by the general formula (4) can be prepared according to the method described in the specification of International Publication No. 2005 / 42474.
[0263] Hereinafter, Tables 1 to 5 show represen...
Embodiment 1-1
[0657] Preparation of N-(2,6-Dimethyl-4-heptafluoroisopropyl)phenyl 3-nitrobenzamide
[0658] Add 20.0g of 2,6-dimethyl-4-heptafluoroisopropylaniline and 11.0g of pyridine to 100ml of tetrahydrofuran, stir at room temperature, and slowly drop 13.0g dissolved in 20ml of tetrahydrofuran into the resulting solution 3 -Nitrobenzoyl chloride. After stirring at room temperature for 10 hours, ethyl acetate and water were added to the reaction solution. After the liquid separation operation, the organic layer was separated and dried with anhydrous magnesium sulfate. The solution was filtered, the filtrate was collected, the solvent was distilled off under reduced pressure, and the obtained residue was washed with a hexane-diisopropyl ether mixed solvent to obtain 26.0 g of the target product as a white solid (yield 85%).
[0659] 1 H NMR(CDCl 3 , Ppm) δ 2.33 (6H, s), 7.37 (2H, s), 7.68 (1H, s), 7.72 (1H, t, J = 8.1 Hz), 8.28 (1H, d, J = 8.1 Hz) , 8.44 (1, H, dd, J = 1.2, 8.1 Hz), 8.75 (1H...
Embodiment 1-2
[0661] Preparation of N-(2,6-Dimethyl-4-heptafluoroisopropyl)phenyl 3-aminobenzamide
[0662] Add 0.90g of N-(2,6-dimethyl-4-heptafluoroisopropyl)phenyl 3-nitrobenzamide and 1.56g of stannous chloride anhydrous to 25ml of ethanol, and stir at room temperature Add 2ml of concentrated hydrochloric acid to the resulting solution and stir at 60°C for 1 hour. After returning to room temperature, the reaction solution was poured into water, and potassium carbonate was used for neutralization. Ethyl acetate was added and the insoluble matter was filtered off, then the organic layer was separated and dried over anhydrous magnesium sulfate. This solution was filtered, the filtrate was collected, the solvent was distilled off under reduced pressure, and the obtained residue was washed with hexane to obtain 0.44 g of the target product as a white solid (yield 53%).
[0663] 1 H-NMR(CDCl 3 , Ppm) δ 2.34 (6H, s), 3.87 (2H, broad), 6.86-6.89 (1H, m), 7.20-7.35 (6H, m)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com