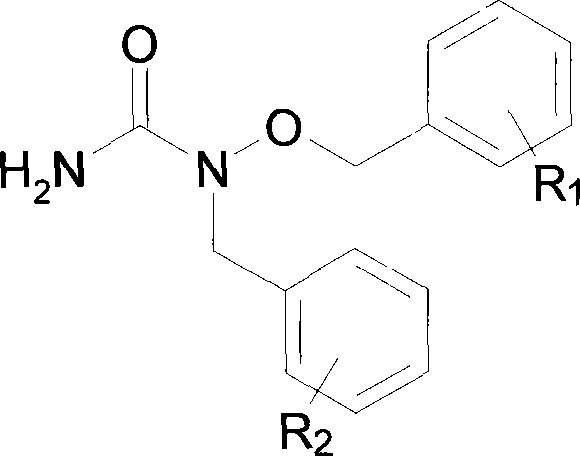
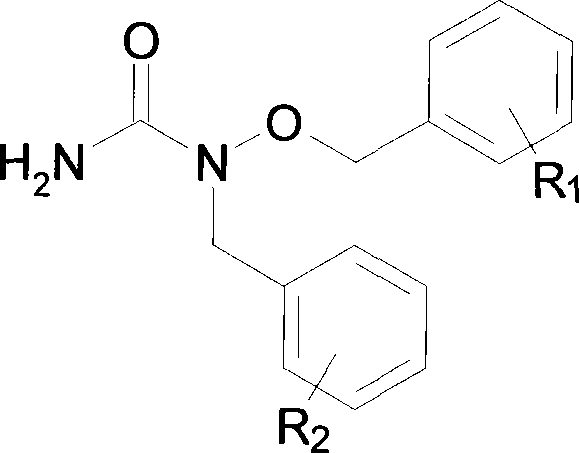
Synthesis of N-benzyl-N-benzyloxy urea
A benzyloxyurea and benzyl technology, applied in the field of synthesis of hydroxyurea derivatives, can solve the problems of small molecular weight of HU, large toxic and side effects of hydroxyurea, low bioavailability, etc., and achieve improved bioavailability and reduced toxic and side effects , the effect of enhanced activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1: Synthesis of N-benzyl-N-benzyloxyurea: Take 2.16g benzyloxyurea and dissolve it in 80ml methanol, add 0.95gKOH, heat to reflux, slowly add dropwise 20ml of methanol solution of 1.60ml benzyl chloride , stirred and refluxed for 16 hours, tracked the reaction with TLC (developing agent: chloroform: acetone=5:1, then sprayed ferric chloride solution, no blue spots were the reaction end point), after the reaction was complete, the reaction solution was reduced at 35 ° C. Pressure distillation, the solid residue was extracted with ether, the extract was concentrated under reduced pressure, and then passed through a silica gel column, the mobile phase was acetone: chloroform = 1: 4, the same point was collected, evaporated to dryness under reduced pressure, and then used acetone: n-hexane = 5: 1.5 recrystallized to obtain 1.07 g of white crystals. m.p = 97-98°C.
Embodiment 2
[0012] Example 2: Synthesis of N-(4-methylbenzyl)-N-(4-methylbenzyloxy)urea: get 2.34g2, and dissolve 4-methylbenzyloxyurea in 80ml methanol, add 0.95gKOH, heated to reflux, slowly added dropwise 20ml of methanol solution of 1.8ml p-methylbenzyl chloride, stirred and refluxed for 17 hours, tracked the reaction with TLC (developing agent: chloroform: acetone=5:1, then sprayed ferric chloride solution , no blue spot is the reaction end point), after the reaction is complete, the reaction solution is distilled under reduced pressure at 35°C, the solid residue is extracted with ether, the extract is concentrated under reduced pressure, and then passed through a silica gel column, the mobile phase is acetone:chloroform=1: 4. The same points were collected, evaporated to dryness under reduced pressure, and then recrystallized with acetone:n-hexane=5:1.5 to obtain 1.26 g of white crystals. m.p = 128-130°C.
Embodiment 3
[0013] Example 3: Synthesis of N-(4-methylbenzyl)-N-(4-methylbenzyloxy)urea: get 2.5g3, and dissolve 4-methoxybenzylurea in 80ml methanol, add 0.95gKOH, heated to reflux, slowly added dropwise 20ml of methanol solution of 2.04ml p-methoxybenzyl chloride, stirred and refluxed for 19 hours, tracked the reaction with TLC (developing agent: chloroform: acetone=5:1, then sprayed ferric chloride solution, no blue spots are the reaction end point), after the reaction is complete, the reaction solution is distilled under reduced pressure at 35°C, the solid residue is extracted with ether, the extract is concentrated under reduced pressure, and then passed through a silica gel column, the mobile phase is acetone:chloroform=1 : 4, the same point was collected, evaporated to dryness under reduced pressure, and then recrystallized with acetone: n-hexane = 5: 2 to obtain 1.48 g of white crystals. m.p = 99-101°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com