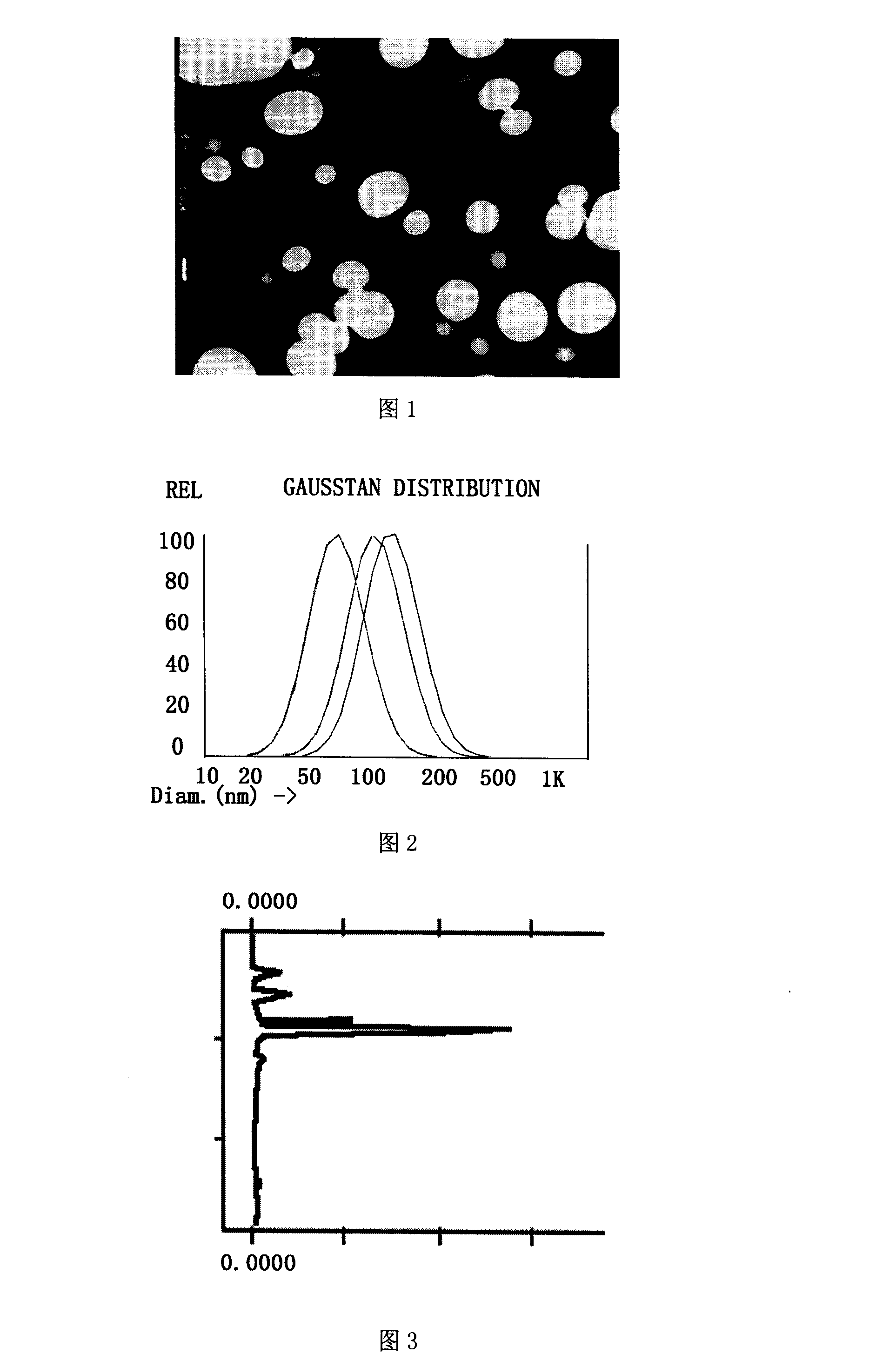
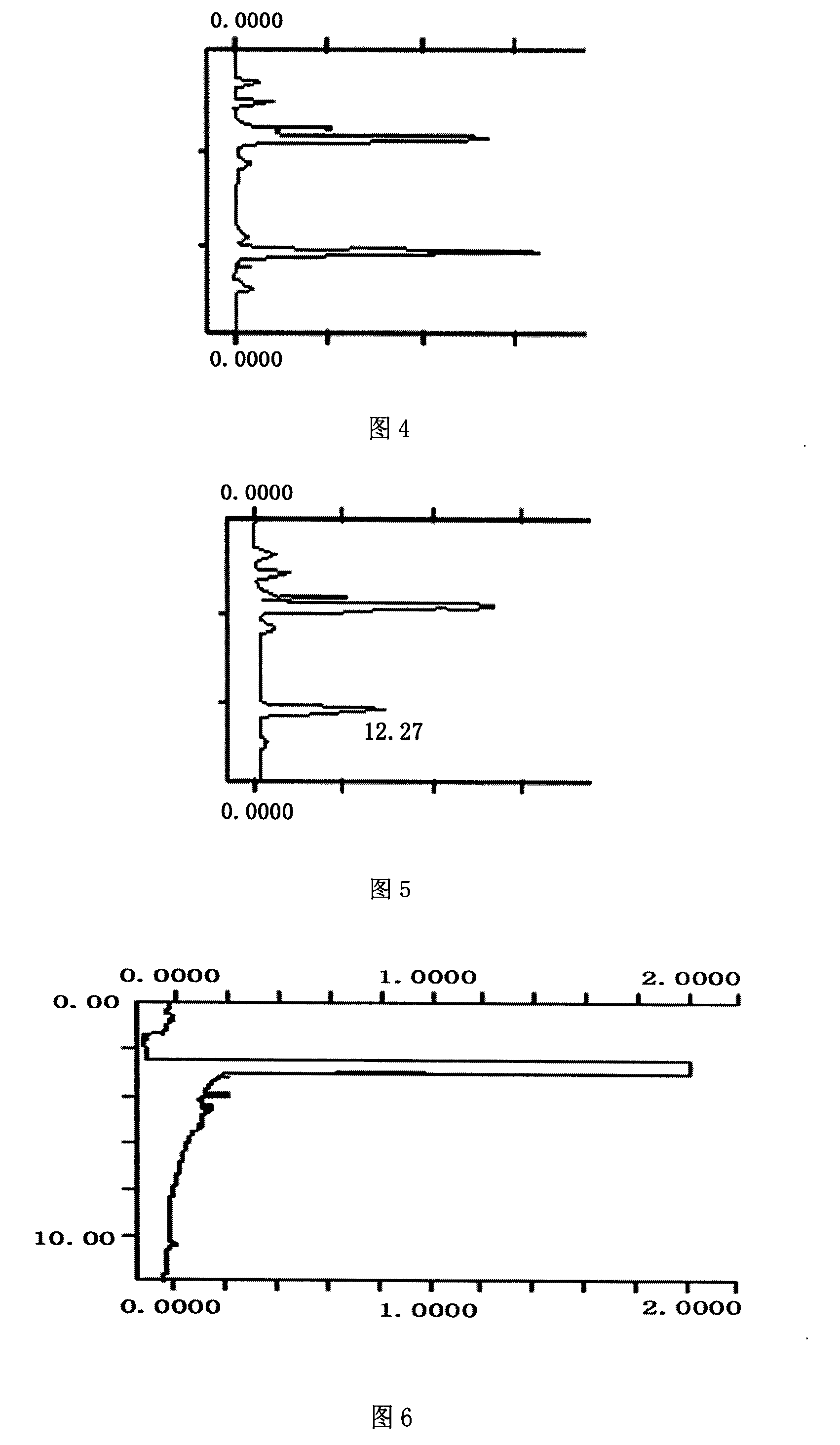
Tanshinone IIA microemulsions and preparing method thereof
A technology of tanshinone and emulsion preparations, applied in the field of tanshinone IIA microemulsion preparations and preparations, can solve the problems of patients with skin rashes, lack of sustained release and targeting, and pain sensation, and achieve thermodynamic stability, transparent appearance, and bioavailability high degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] Get sterilized Tanshinone IIA (Xi'an Baosai Natural Products Technology Co., Ltd., content ≥ 98%, batch number BSCD051011), phospholipids for injection (degussa, Epikuron 170 batch number 159070), glycerin for injection (Hunan Erkang Pharmaceutical Co., Ltd., batch number 20070101 ), F for injection 68 (Shenyang Yaoda Pharmaceutical Co., Ltd., batch number 060510), ethyl oleate (Tianjin Guangfu Fine Chemical Research Institute, batch number 20050128, specification Tianjin Q / HGNK204-2000), water for injection (Tianjin Baite Medical Supplies Co., Ltd., batch number C0607212A) is the raw material, and the mass percentages of each raw material in the total weight are tanshinone IIA 0.6% to 0.8%, phospholipids for injection 0.6% to 1.8%, glycerin for injection 2.5% to 3%, F for injection 68 1.5% to 3%, ethyl oleate 11% to 25%, the balance of water for injection, the sum of the raw materials used is 100%.
[0031] b. using ultrasonic method to dissolve tanshinone IIA in ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com