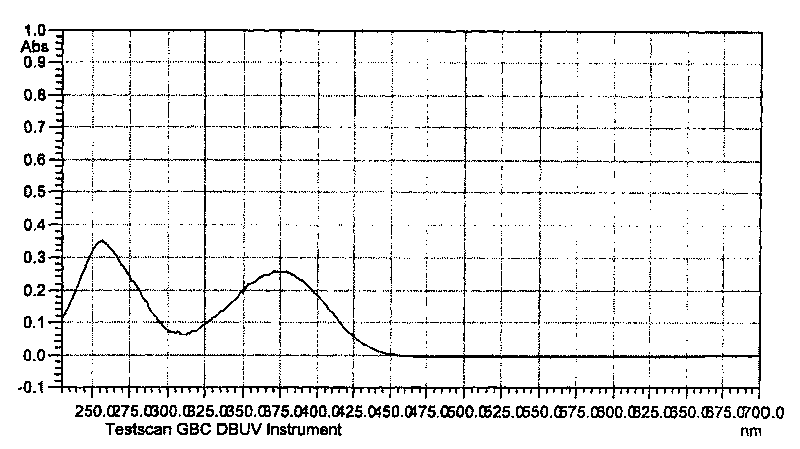
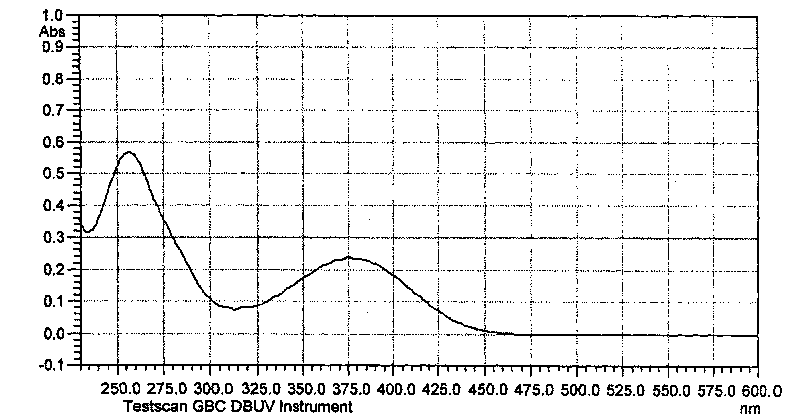
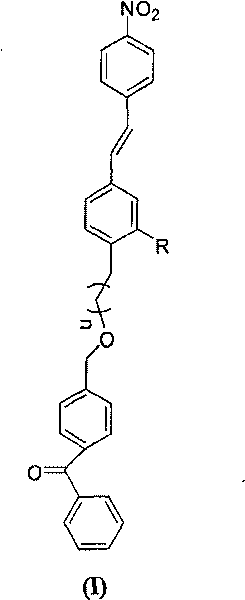
Non-conjugated p-nitro diphenyl ethylene dye containing benzophenone via ether linkage as well as synthesis and uses thereof
A technology of nitrostilbene and benzophenone, which is applied in the direction of styrene-based dyes, nitro/nitroso dyes, organic dyes, etc., can solve the problems of low initiation efficiency and short absorption wavelength, and achieve raw material The effect of convenient source, wide absorption band, and easy source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of (p-nitro-stilbene)-(4-methylbenzophenone)-ether
[0045] The synthesis proceeds in three steps:
[0046] (1) 4-nitro-4'hydroxy-stilbene
[0047] The ratio of 1.2g p-Hydroxybenzaldehyde and 2.26g p-nitrophenylacetic acid (molar ratio 0.8: 1) is mixed in three-necked ground flasks, add condensing device, then add 1.06g of hexahydropyridine (molar ratio 1: 1.25), reacted under heating at 110°C for 6 hours, dissolved in ethanol for recrystallization, and filtered to obtain crystals with a yield of 66%, and set aside;
[0048] (2) Synthesis of 4-bromomethylbenzophenone
[0049] 1.0g 4-methylbenzophenone is mixed with 0.91g N-bromosuccinimide (NBS) (molar ratio 1: 1) in a three-necked ground-necked flask, and a condensing device is added. 4 Reflux for 6 hours, filter, recrystallize the solid in benzene / cycloethane, filter to obtain crystals, the yield is 55%, and set aside;
[0050] (3) (p-nitrostilbene)-(4-methylbenzophenone)-ether
[0051] With step (1), the...
Embodiment 2
[0053] Synthesis of (4-nitro-3'-methylstilbene)-(4-methylbenzophenone)-ether
[0054] The synthesis proceeds in three steps:
[0055] (1) Synthesis of 4-nitro-3'-methyl, 4'-hydroxyl-stilbene
[0056] The ratio of 1.36g 3-methyl-4-hydroxybenzaldehyde and 2.4g p-nitrophenylacetic acid (molar ratio 0.8: 1) is mixed in a three-necked ground flask, add a condensing device, and then add 1.06g of hexahydro Pyridine (molar ratio is 1:1.25), reacted under heating at 120°C for 6 hours, dissolved in ethanol for recrystallization, filtered to obtain crystals, yield 67%, and set aside;
[0057] (2) Synthesis of 4-bromomethylbenzophenone
[0058] Synthesis is carried out by the second step in the implementation case 1;
[0059] (3) Synthesis of (4-nitro-3'-methylstilbene)-(4-methylbenzophenone)-ether
[0060] 2.55g of 4-nitro-3'-methylstilbene and 3.3g of 4-bromomethylbenzophenone (molar ratio 1:1.2) prepared in steps (1) and (2) were mixed and added to the third port Mix in a ground-n...
Embodiment 3
[0062] Synthesis of (p-nitrostilbene)-[3,4-bis(methylbenzophenone)]-ether
[0063] The synthesis proceeds in three steps:
[0064] (1) p-Nitro-3',4'-dihydroxystilbene
[0065] The ratio of 1.38g 3,4-dihydroxybenzaldehyde and 2.4g p-nitrophenylacetic acid (0.8: 1 in molar ratio) is mixed in three-necked ground flasks, add condensing device, then add 1.06g of hexahydropyridine ( The molar ratio is 1:1.25), reacted under heating at 120°C for 6 hours, dissolved in ethanol for recrystallization, and filtered to obtain crystals with a yield of 67%, which was set aside;
[0066] (2) Synthesis of 4-bromomethylbenzophenone
[0067] Synthesis is carried out by the second step in the implementation case 1;
[0068] (3) Synthesis of (p-nitrostilbene)-[3,4-bis(methylbenzophenone)]-ether
[0069] 2.57g 4-nitro-3', 4'-dihydroxy stilbene and 5.5g 4-bromomethylbenzophenone (molar ratio 1: 2) were mixed with step (1), (2) prepared Add it into a three-necked ground-necked flask and mix it, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com