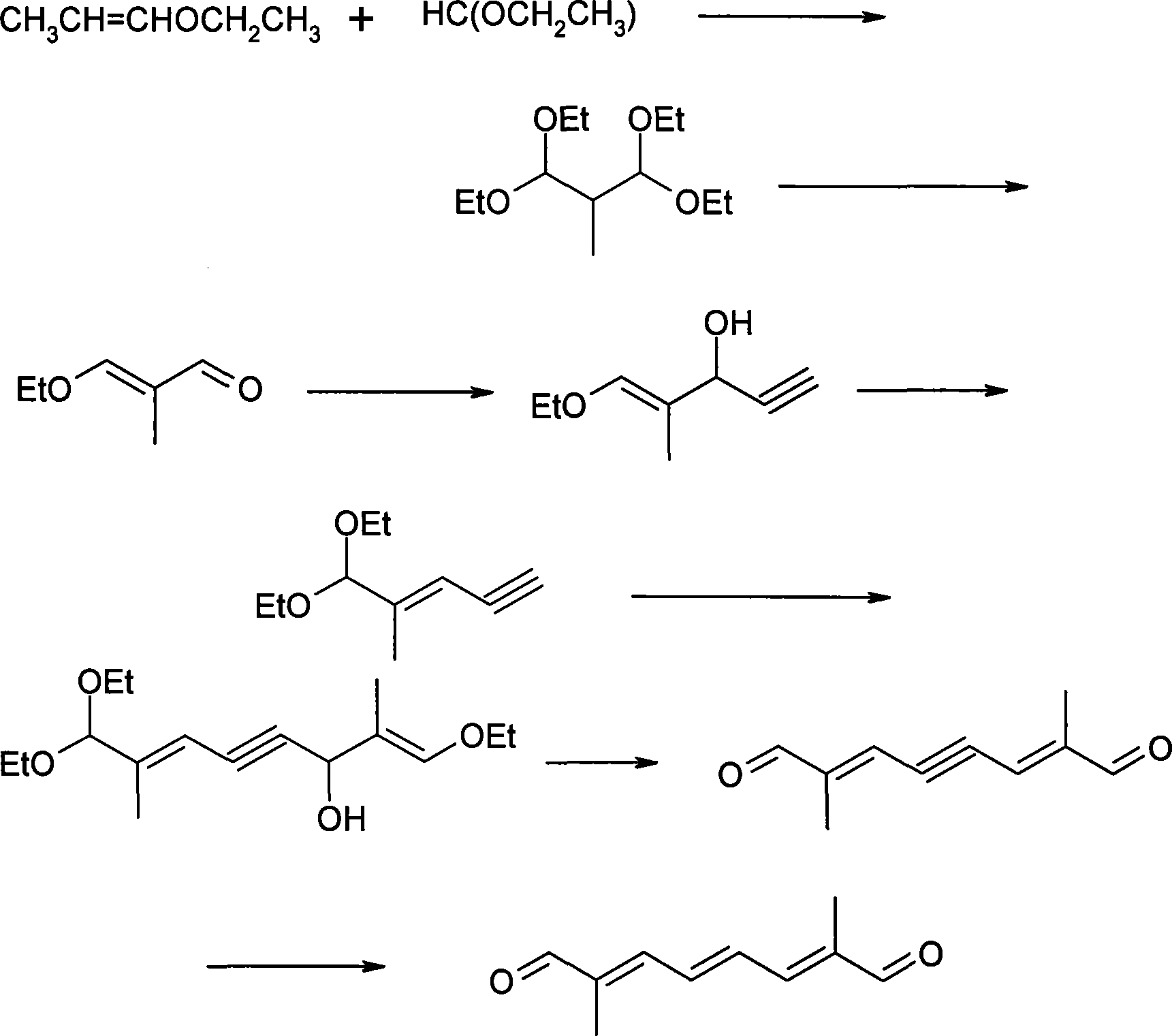
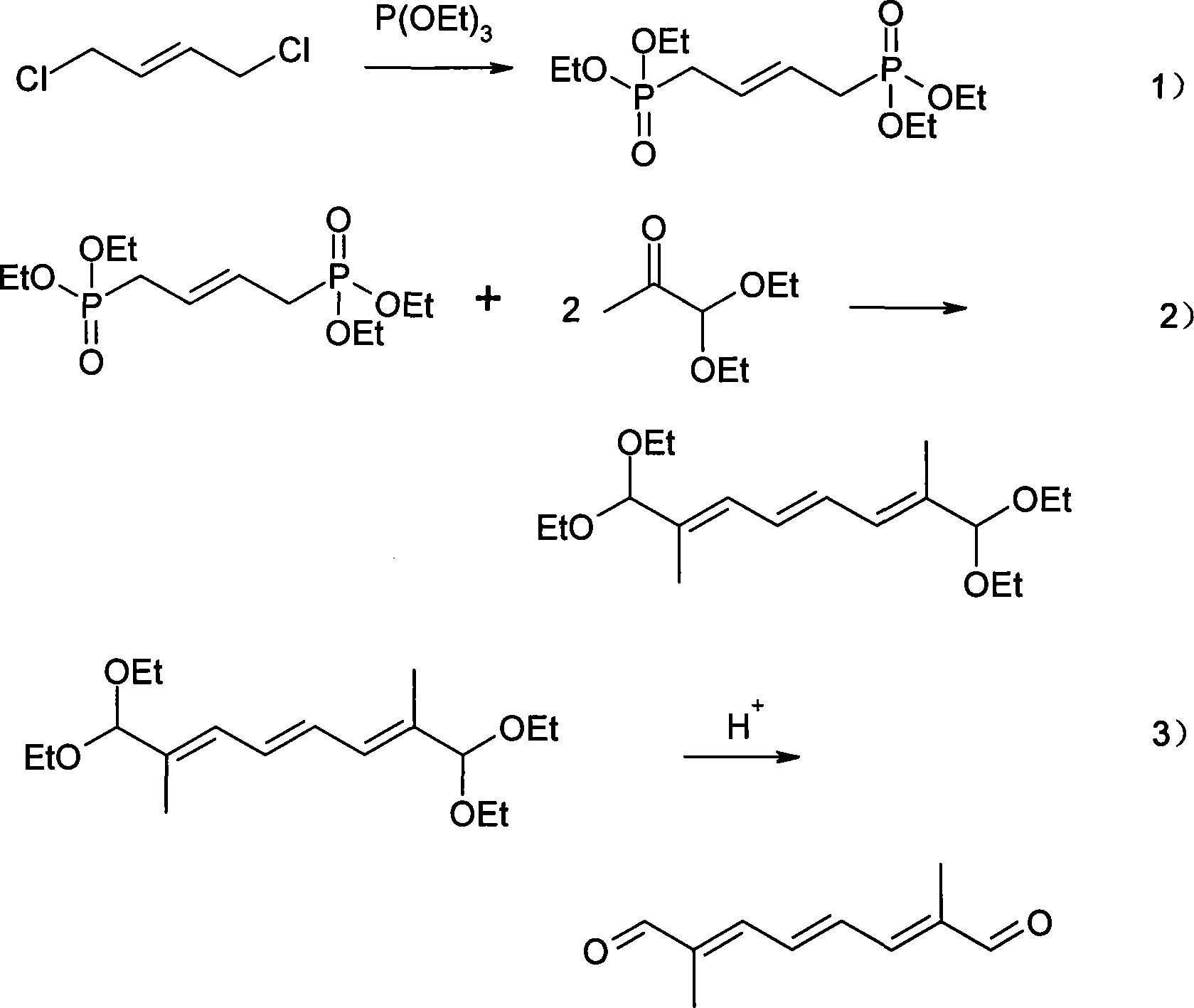
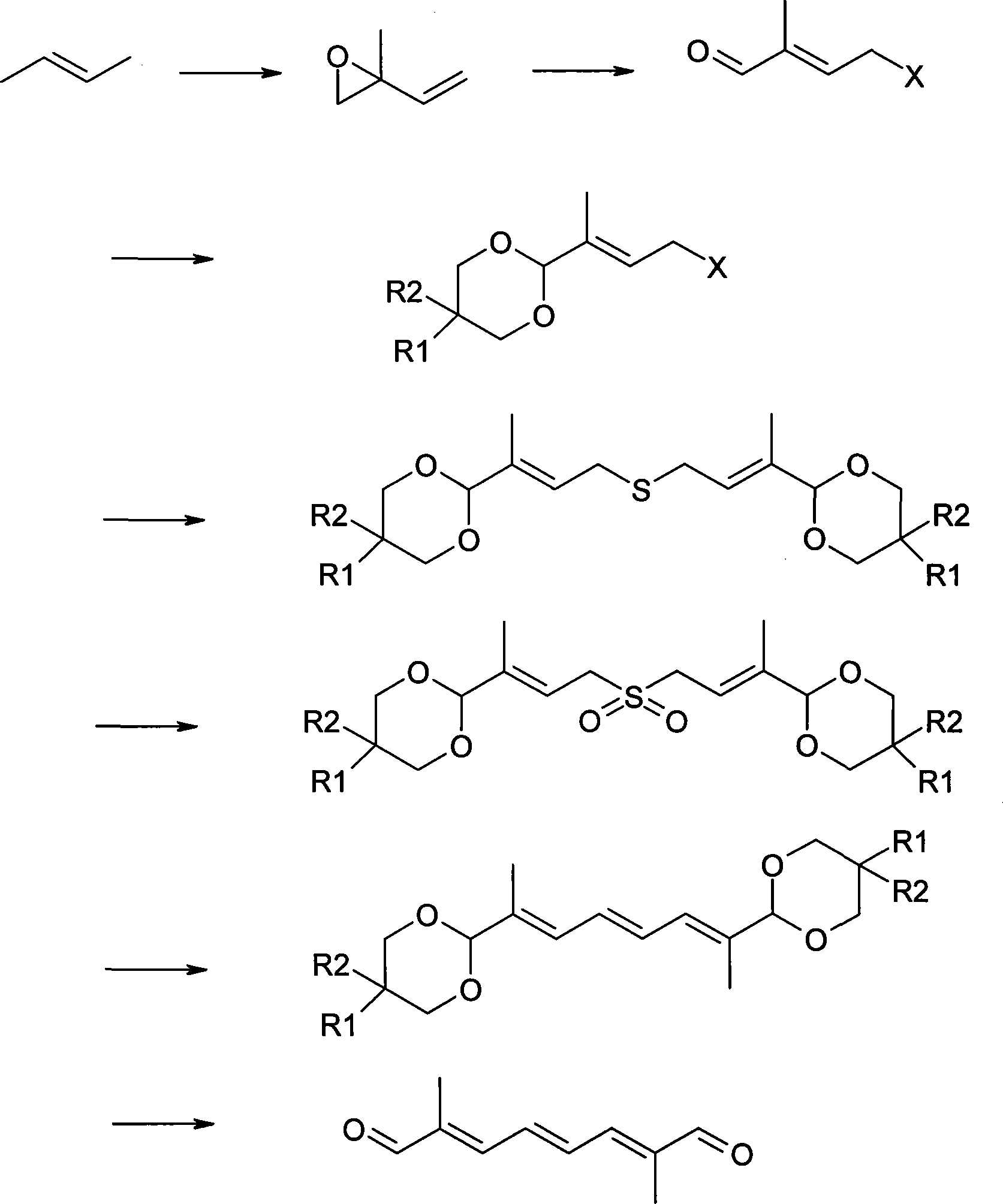
Method for synthesizing 2,7-dimethyl-octa-2,4,6-trienedial
A technology of octatriene and dimethyl, applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problem of high cost of triethyl phosphite, short reaction steps, and long process flow and other problems, to achieve the effects of convenient operation, high total yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 300ml of tetrahydrofuran, 12.0g of magnesium powder, 2.1ml of trans-1,4-dichloro-2-butene with a gas phase content of 99.0%, and 0.1g of iodine into the reactor, heat, control the reaction temperature to 30°C, drop Add 11.3ml of trans-1,4-dichloro-2-butene with a gas phase content of 99.0%, dropwise, heat up to 40°C, keep the temperature for 2 hours, add dropwise 100ml of dimethoxyacetone containing 29.5g Tetrahydrofuran solution, after dripping, heat preservation reaction for 6 hours, cooled to 20°C to obtain the reaction solution; added the reaction solution dropwise to 150ml of dilute sulfuric acid with a concentration of 10% by weight for hydrolysis, the hydrolysis temperature was 0°C, continued to stir for 0.5 hours, and heated up To 30°C, remove tetrahydrofuran under reduced pressure, add 100ml of water, filter, wash the filter cake with water, and dry in vacuum to obtain 2,7-dimethyl-2,4,6-octatriene-1,8-dialdehyde.
Embodiment 2
[0024] Add 300ml of tetrahydrofuran, 12.0g of magnesium powder, 4.2ml of trans-1,4-dichloro-2-butene with a gas phase content of 99.0%, and 0.1g of iodine into the reactor, heat, control the reaction temperature to 35°C, drop Add 22.6ml of trans-1,4-dichloro-2-butene with a gas phase content of 99.0%, dropwise, heat up to 60°C, keep the temperature for 5 hours, add dropwise 400ml of tetrahydrofuran containing 295g of dimethoxyacetone solution, dripping is completed, heat preservation reaction for 12 hours, cooled to 40 ℃ to obtain the reaction solution; the reaction solution is added dropwise to 600ml weight percent concentration and hydrolyzed in 20% dilute hydrochloric acid, the hydrolysis temperature is 20 ℃, continue to stir for 2 hours, and heat up to 50°C, remove tetrahydrofuran under reduced pressure, add 600ml of water, filter, wash the filter cake with water, and dry in vacuum to obtain 2,7-dimethyl-2,4,6-octatriene-1,8-dialdehyde.
Embodiment 3
[0026] Add 300ml of tetrahydrofuran (THF), 12.0g of Mg powder, 5g of trans-1,4-dichloro-2-butene (content: 99.0%), and 0.1g of iodine in a 1000ml flask, heat to initiate, and then control the reaction The temperature is 30-35°C, add the remaining 26.6g of trans-1,4-dichloro-2-butene dropwise, after the drop is complete, raise the temperature to 50°C, keep warm until the Mg powder disappears completely; Add dropwise 200ml of THF solution containing 118g of dimethoxyacetone. After dropping, keep the temperature for 8 hours and cool down to 20°C; dropwise add the reaction solution into 300ml of 10% dilute sulfuric acid for hydrolysis, control the hydrolysis temperature below 20°C, drop Continue to stir for 30 minutes after completion, and a large amount of product precipitates out; the temperature is controlled below 50°C, THF is removed under reduced pressure, 300ml of water is added thereto, filtered, the filter cake is rinsed with a small amount of water, and the wet product is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com