Method for synthesizing trioctyl lemon acid
A technology of trioctyl citrate and citric acid, applied in the field of organic chemical synthesis, can solve the problems of complicated post-processing, difficult refining, serious equipment corrosion, etc., and achieves the effects of small catalyst dosage, no environmental pollution, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The preparation of the first step naphthalenesulfonic acid methylal
[0015] Heat 10g of naphthalene to 160°C, then slowly add 10ml of concentrated sulfuric acid dropwise, then heat and stir at 140°C for 5h, then cool to 100°C, add 2ml of water, dropwise add 10ml of formaldehyde solution, keep heating at 100°C for 4h, to obtain black solid, then add boiling water to wash, filter with suction, and dry at 120°C to obtain methylal of naphthalenesulfonic acid.
[0016] Step 2 esterification reaction
[0017] Add 0.1mol citric acid, 0.45mol n-octanol, and 10ml water-carrying agent toluene into a 100mL three-necked bottle equipped with electromagnetic stirring, a thermometer, a reflux condenser, and a water separator, and heat and stir. After the citric acid is completely dissolved, add 0.3g of naphthalenesulfonic acid methylal is heated under reflux and stirred to separate the water generated by the reaction from the water separator, and the reaction is stopped when there i...
Embodiment 2-4
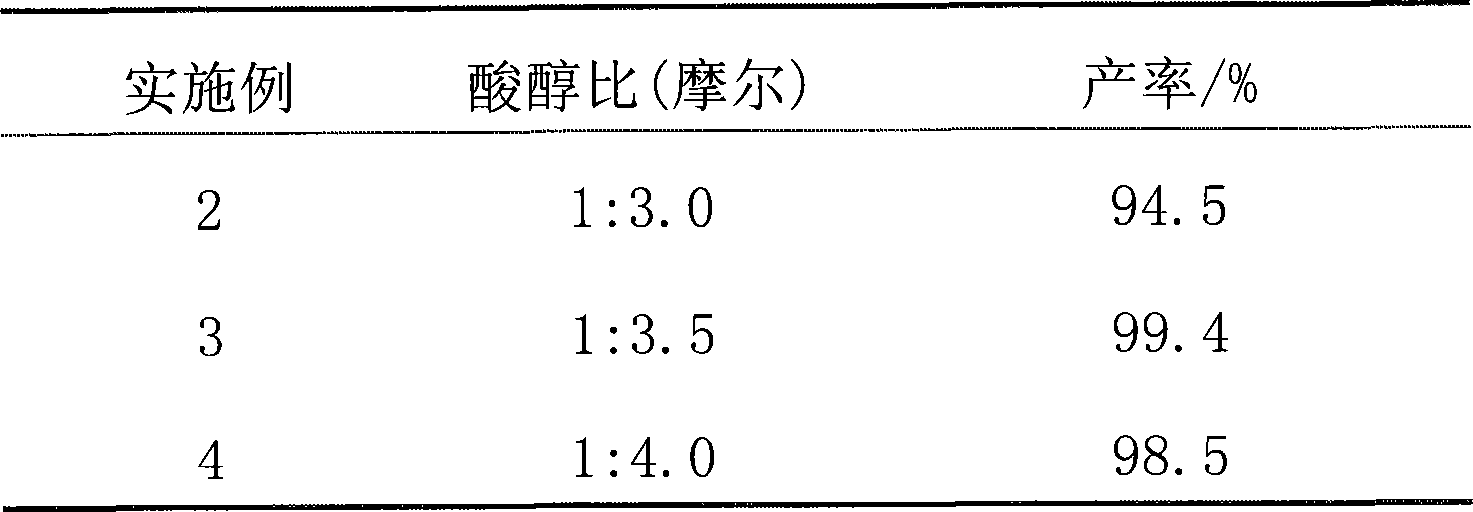
[0022] Except for the following differences, all the other are the same as in Example 1. The amount of naphthalenesulfonic acid methylal is 0.3 g, the amount of citric acid is 0.1 mol, and the ratio of n-octanol in Table 1 is used for 4 hours.
[0023] Table 1
[0024]
Embodiment 5-7
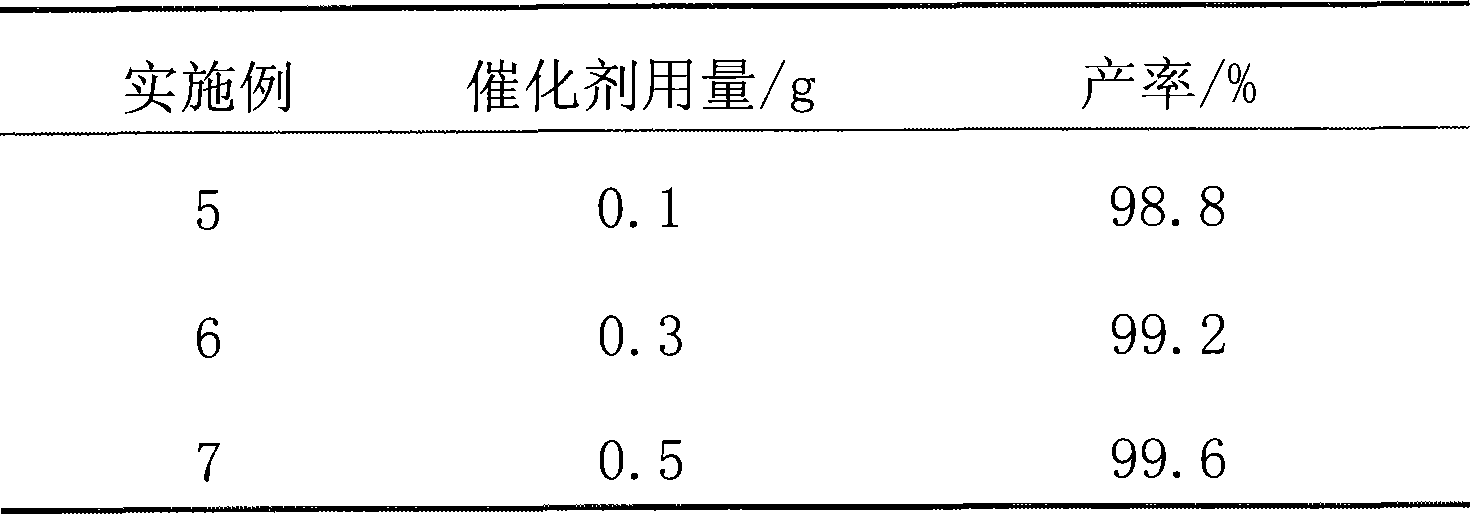
[0026] Except following difference, all the other are identical with embodiment 1, and the consumption of naphthalenesulfonic acid methylal is respectively by table 2.
[0027] Table 2
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com