Polybutylene terephthalate and process for production thereof
A technology of polybutylene terephthalate and terephthalic acid, applied in the field of polybutylene terephthalate and its manufacture, can solve the problem of reduction of impurities and haze, deactivation, polyterephthalate Butylene glycol diformate has problems such as deterioration of color tone, and achieves the effect of excellent moldability and reduction of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
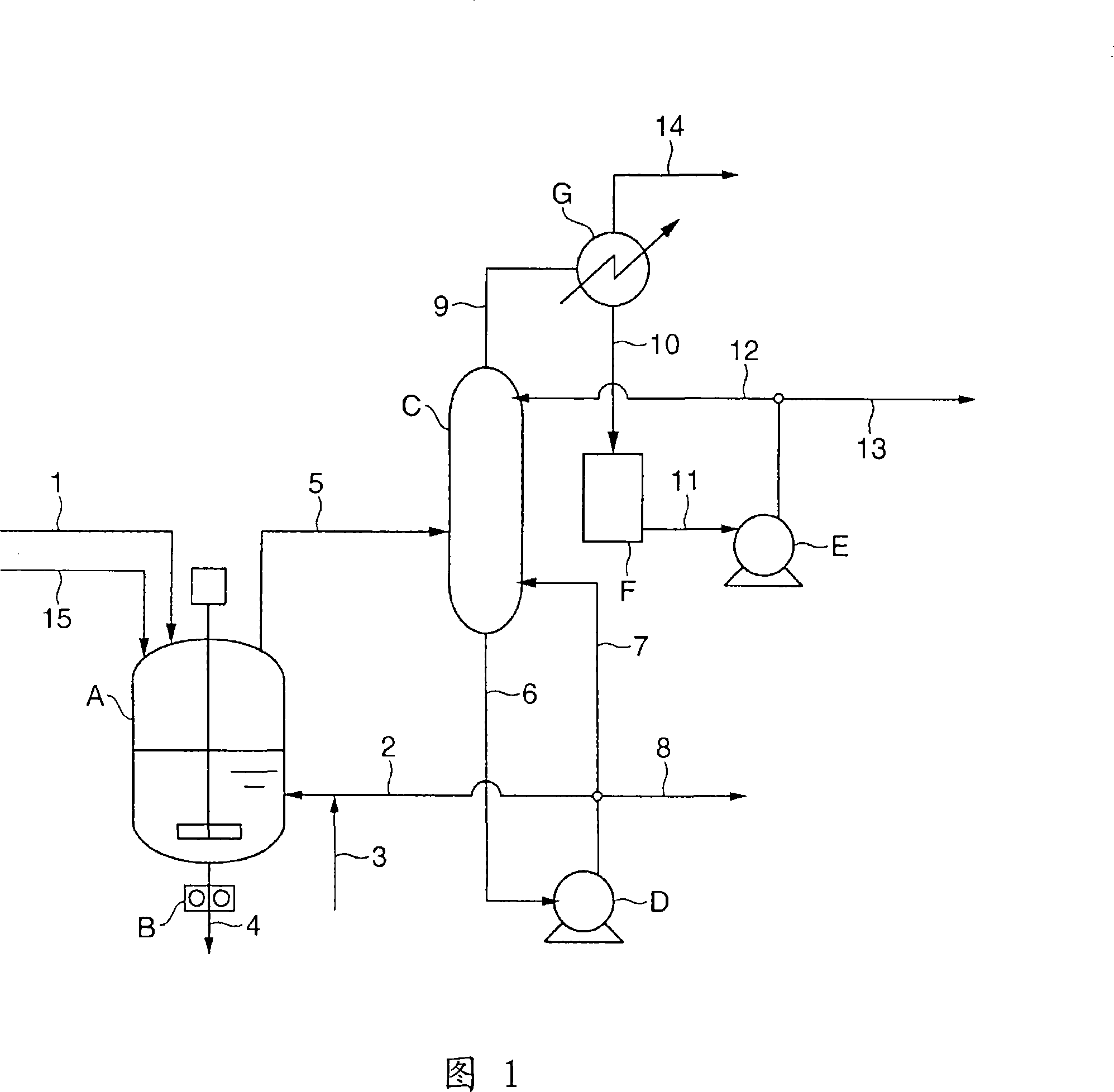
[0183] Through the esterification step shown in FIG. 1 and the polycondensation step shown in FIG. 2 , PBT is produced in the following manner. First, a 60°C slurry mixed with 1.80 mol of 1,4-butanediol per 1.00 mol of terephthalic acid was continuously supplied at 40 kg / h from the slurry preparation tank through the raw material supply line (1). Into a reactor (A) for esterification with a propeller-type agitator filled in advance with a PBT oligomer having an esterification rate of 99%. Simultaneously, the bottom component (98% by weight or more is 1,4-butanediol) of the rectification column (C) at 185° C. is supplied at 18.4 kg / h from the recirculation line (2), and the titanium catalyst is supplied from the titanium catalyst supply line (3 ) was supplied at 127 g / h as a 65° C. tetrabutyl titanate 6.0% by weight 1,4-butanediol solution as a catalyst. The water content in this catalyst solution was 0.2% by weight. .
[0184] The internal temperature of the reactor (A) is ...
Embodiment 2
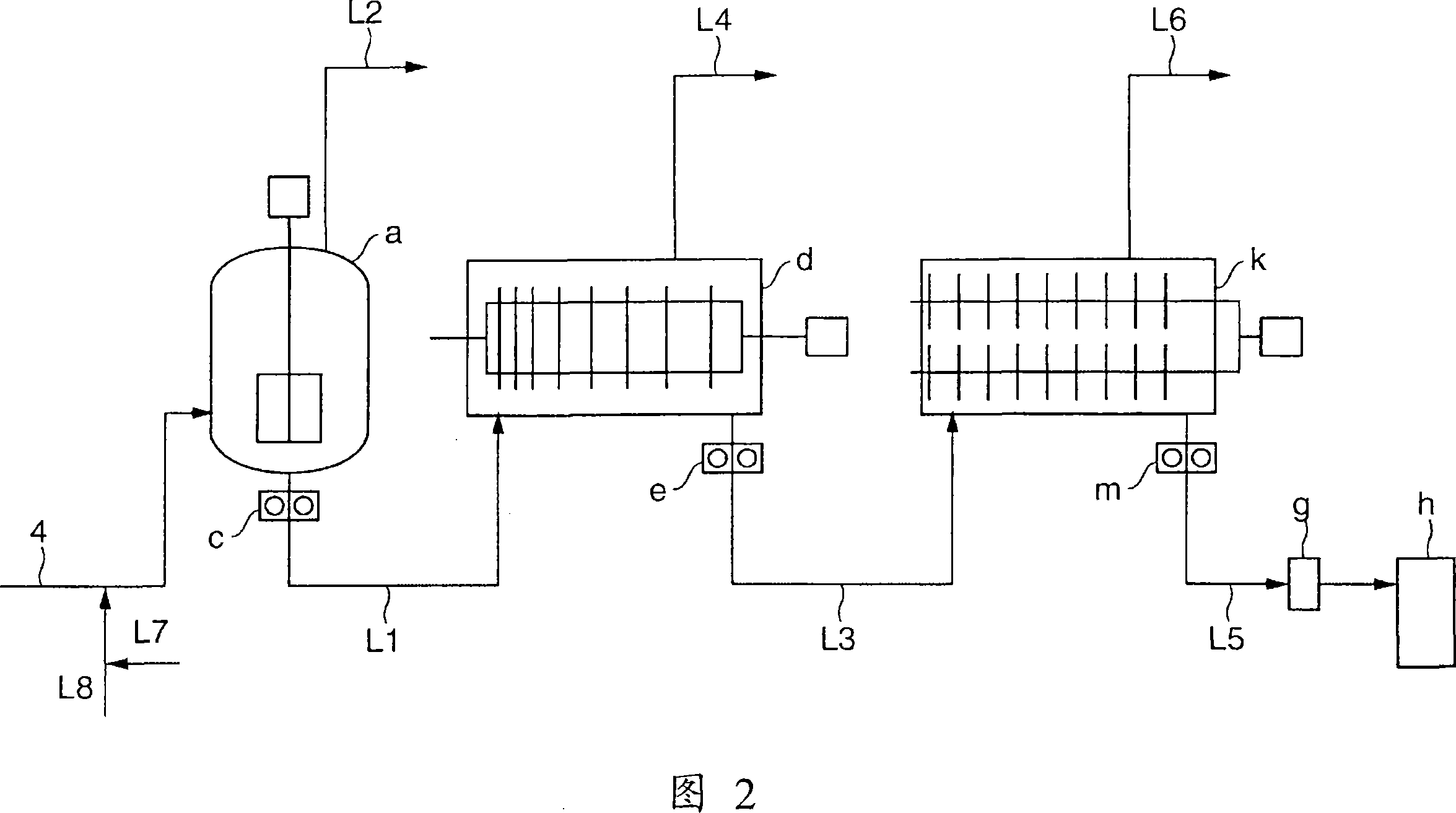
[0190] Esterification reaction was carried out in the same manner as in Example 1, except that the magnesium acetate tetrahydrate salt solution of the amount shown in Table 1 was supplied from the line (15). After the system stabilized, the esterification rate of the oligomers collected at the outlet of the reactor (A) was 96.5%. On the other hand, the supply amount of magnesium acetate tetrahydrate supplied to the oligomer extraction line (4) is as shown in Table 1, and the concentration of magnesium acetate tetrahydrate supplied to the line (4) was 0.88% by weight. The condition of the 1st polycondensation reactor (a) is identical with embodiment 1; The internal temperature of the 2nd polycondensation reactor (d) is 240 ℃, and pressure is 160Pa; The internal temperature of the 3rd polycondensation reactor (k) is 243 ℃, Except for this, polycondensation reaction was carried out in the same manner as in Example 1. Table 1 shows the analytical values of the obtained PBT. It...
Embodiment 3
[0192] Esterification reaction was carried out in the same manner as in Example 1, except that the magnesium acetate tetrahydrate salt solution in the amount shown in Table 1 was supplied from the line (15) and the average residence time was 3.4 hours. After the system stabilized, the esterification rate of the oligomers collected at the outlet of the reactor (A) was 95.4%. On the other hand, the supply amount of magnesium acetate tetrahydrate supplied to the oligomer extraction line (4) and the conditions of the first polycondensation reactor (a) were the same as in Example 1; the internal temperature of the second polycondensation reactor (d) The polycondensation reaction was carried out in the same manner as in Example 1 except that the internal temperature of the third polycondensation reactor (k) was 241° C., and the pressure was 160 Pa. The internal temperature was 244° C. Table 1 shows the analytical values of the obtained PBT. It is excellent in color tone and trans...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com