Application of ionic liquid used as solvent in benzene and cyclohexane extraction, rectification and separation
An ionic liquid solvent and ionic liquid technology, which is applied in distillation purification/separation, organic chemistry, bulk chemical production, etc., can solve the problems of complex separation process, equipment investment and high energy consumption of separation, and achieve high chemical stability and wide Effect of liquid temperature range, process and operational simplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
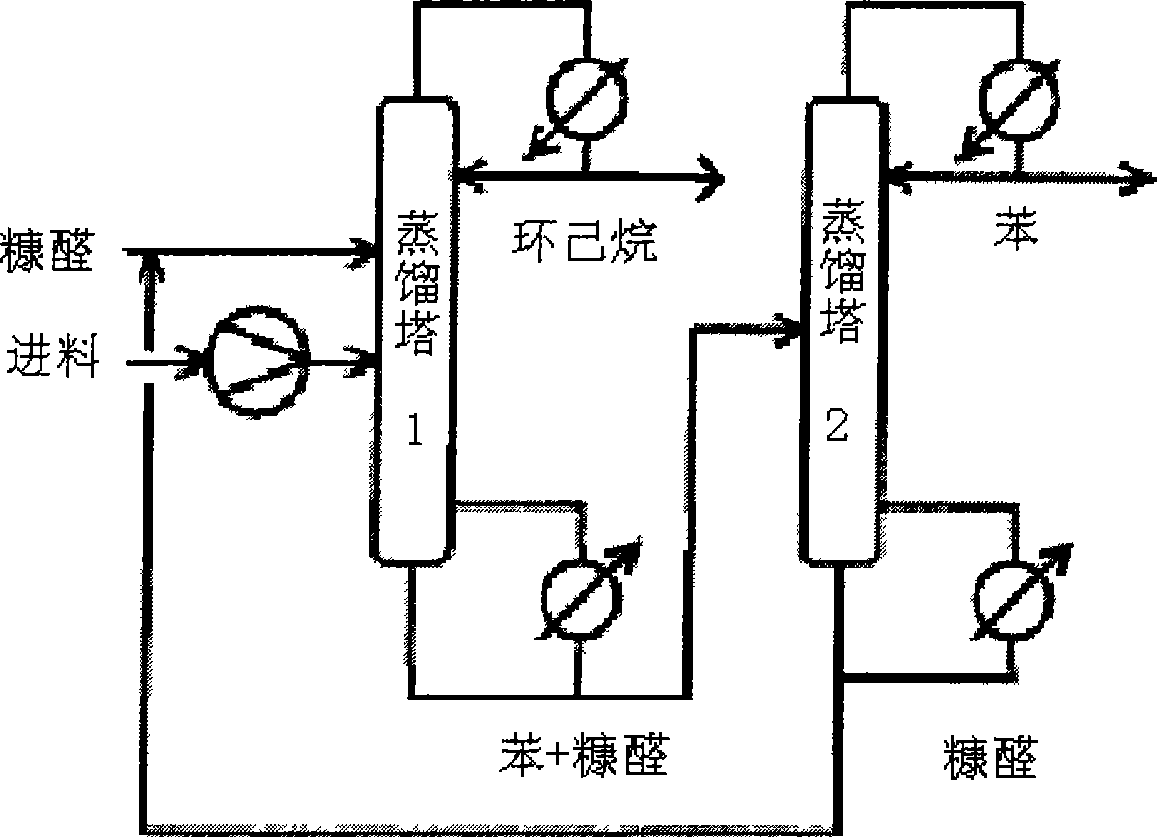
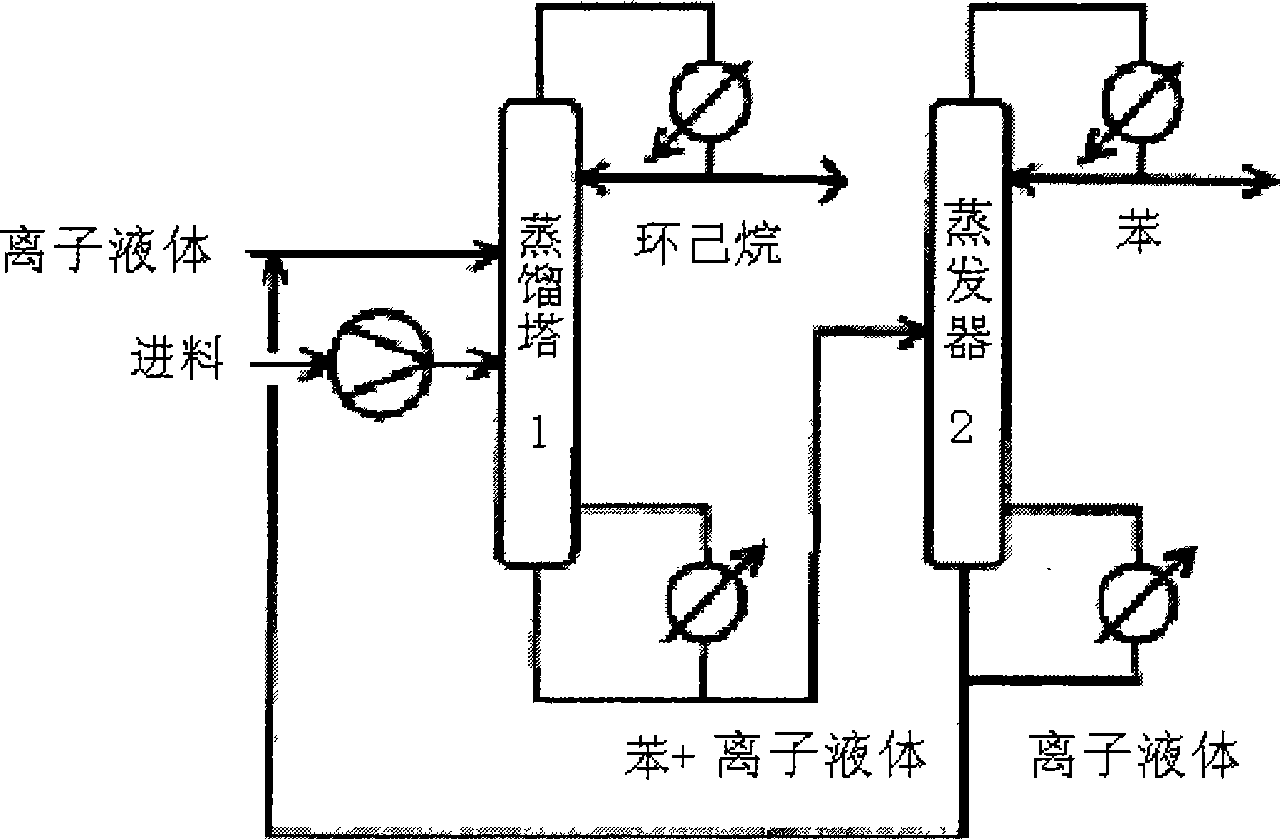
Image
Examples
Embodiment 1
[0043] Utilize ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate as extractant to join in the mixture of benzene and cyclohexane (benzene molar content is 10%~90%), its addition has great influence on the relative volatility of the system The impact can be seen in Table 3.
[0044] Table 3: Effect of adding ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate on relative volatility
[0045]
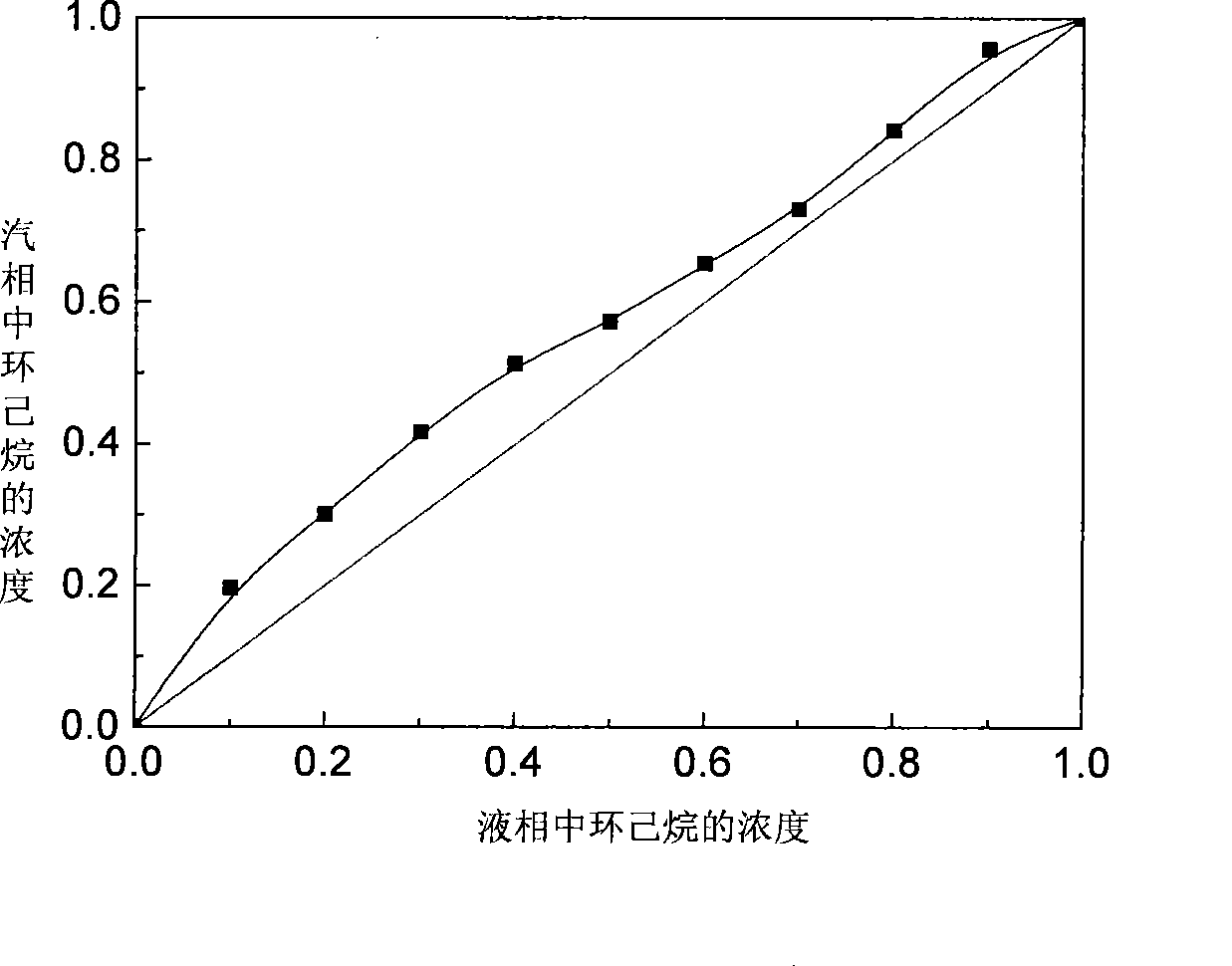
[0046] According to the experiment, the relative volatility of cyclohexane / benzene is improved to a certain extent when the molar content of ionic liquid is 10%~30%. The effect of the vapor-liquid equilibrium curve is more significant, see the attached image 3 , in the whole concentration range, the vapor pressure of cyclohexane is higher than that of benzene, so that the volatility of cyclohexane is greater than that of benzene. In the concentration range of benzene from 10% to 90%, cyclohexane The relative volatility of p-benzene exceeds 1, realizing the use of ...
Embodiment 2
[0048] Utilize ionic liquid 1-butyl-3-methylimidazolium bromide as extractant to add in the mixture of benzene and cyclohexane (benzene molar content is 10%~90%), the influence of its adding amount on the relative volatility of the system See Table 4.
[0049] The impact on relative volatility after adding ionic liquid 1-butyl-3-methylimidazolium bromide salt in table 4
[0050]
[0051]
[0052] As can be seen from the above table, after adding 1-butyl-3-methylimidazolium bromide with 20% molar concentration of ionic liquid, the molar content of cyclohexane is within the range of 10% to 90%. The relative volatility has been improved to a certain extent. Except that the relative volatility is 0.99 when the cyclohexane content is 0.7, the relative volatility is higher than 1 at other concentrations. It can be seen that this kind of ionic liquid can also be added to benzene and cyclohexane as an extraction agent. Extractive distillation and separation in hexane.
Embodiment 3
[0054] Utilize ionic liquid 1-butyl-3-methylimidazolium bromide as extractant to add in the mixture of benzene and cyclohexane (benzene molar content is 10%~90%), the influence of its adding amount on the relative volatility of the system See Table 5.
[0055] The impact on relative volatility after adding ionic liquid 1-butyl-3-methylimidazolium bromide in table 5
[0056]
[0057]
[0058] As can be seen from the above table, after adding ionic liquid 1-butyl-3-methylimidazolium bromide, the relative volatility of cyclohexane / benzene is improved in various degrees, except that the initial molar content of benzene in the raw material is When the relative volatility is less than 1 at 0.8, the other molar contents are all greater than 1, which provides a basis for the separation of benzene and cyclohexane by extractive distillation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com