Preparation for paclitaxel and derivatives thereof
A derivative, paclitaxel technology, applied in the field of preparation of paclitaxel and its derivatives, can solve problems that are not involved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Preparation of Paclitaxel by Catalytic Acetylation of 10-Deacetyl-Paclitaxel Using Various IIIB Metal Element Salts
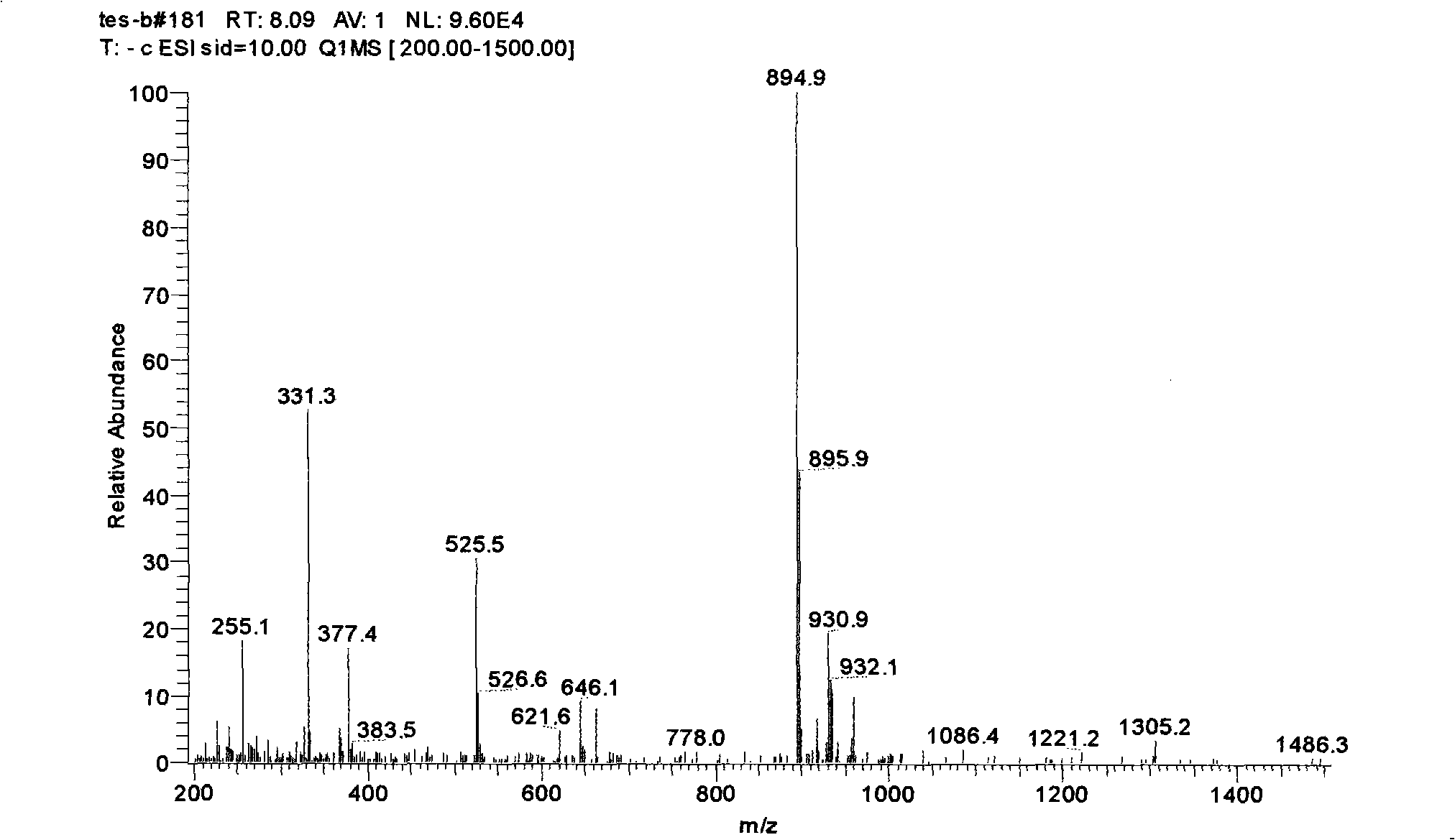
[0054] Weigh 10-deacetyl-paclitaxel (content 62%, LC-MS spectrogram see figure 1 ) sample 100mg was dissolved in 1ml reaction solvent, and the salt of metal elements of group IIIB of catalytic equivalent was added, and after mixing well, about 3 equivalents of acetic anhydride was slowly added dropwise, stirred at room temperature, and TLC detected that 10-deacetyl-paclitaxel was completely converted 2'-O-acetyl-paclitaxel (LC-MS spectrum see figure 2 ), add 1ml saturated NaHCO 3 The solution was extinguished, and then the reaction was moved to an ice-water bath (0° C.), and 1 ml of 30% hydrogen peroxide was slowly added dropwise. After 1.5 h, TLC detected that the reaction was complete. After the reaction, the feed liquid was extracted with ethyl acetate, dried over anhydrous sodium sulfate, and vacuum rotary evaporated to obtain paclitaxel...
Embodiment 2
[0057] Example 2 Preparation of paclitaxel from 10-deacetyl-paclitaxel by various hydrolysis methods
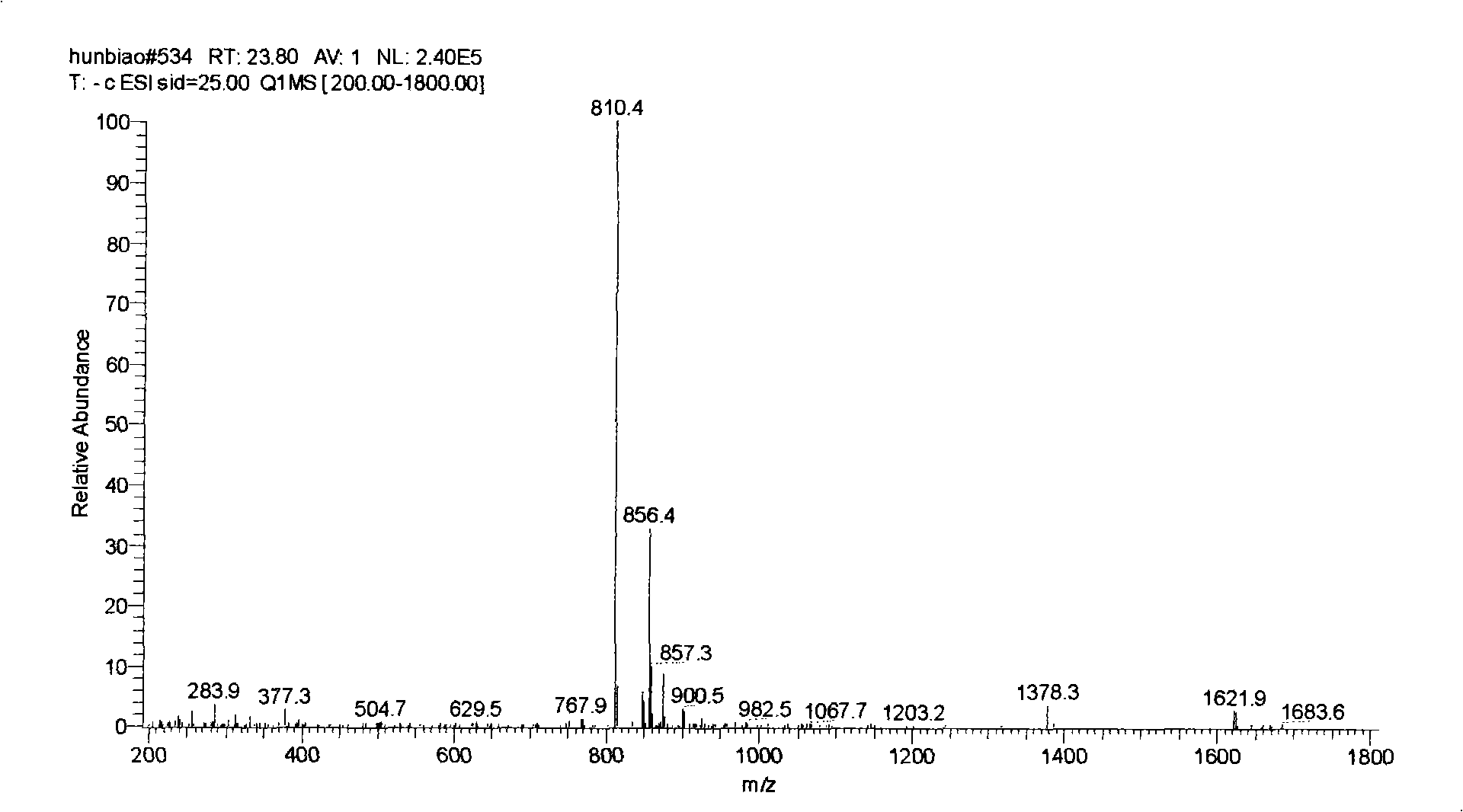
[0058] Weigh 10-deacetyl-paclitaxel (content 62%, LC-MS spectrogram see figure 1 ) sample 100mg was dissolved in 1ml reaction solvent, and catalytic equivalent of cerium chloride was added, after mixing well, about 3 equivalents of acetic anhydride was slowly added dropwise, after stirring at room temperature for 2 hours, 1ml of water was added to extinguish the reaction, and then an appropriate amount of over Oxides and alkaline substances react at room temperature, and TLC detects that the reaction is complete. After the reaction, the feed liquid was extracted with ethyl acetate, dried over anhydrous sodium sulfate, and vacuum rotary evaporated to obtain paclitaxel (see the LC-MS spectrogram image 3 ). Various reaction solvents, peroxides, alkaline substances and their corresponding dosages, reaction time and the yield of the target product paclitaxel are shown in Table ...
Embodiment 3
[0062] Example 3 "One-pot method" to prepare cephalomannine from 10-deacetyl-cephalomannine
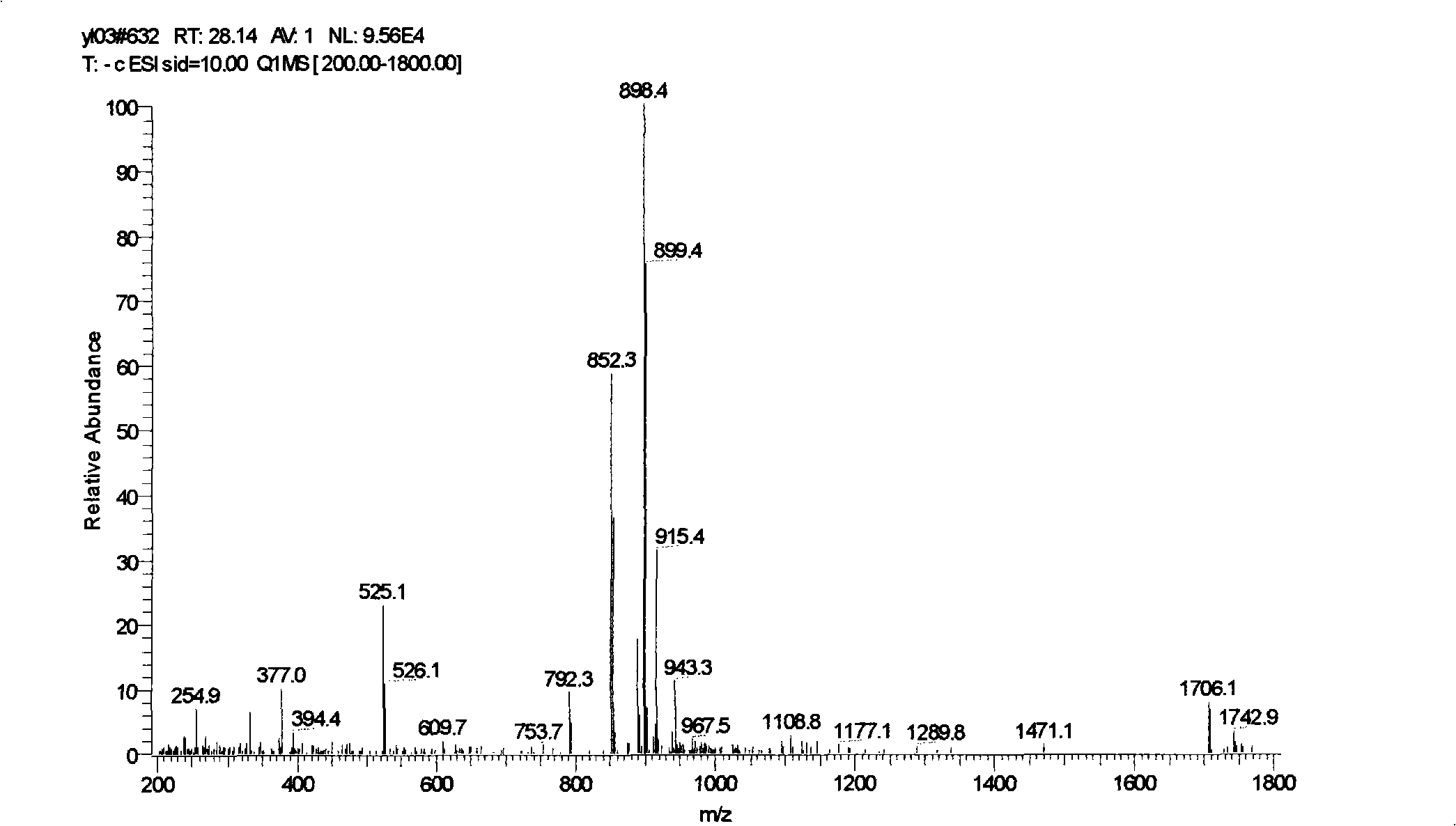
[0063] Weigh 10-deacetyl-cephalomannine (content 85%, LC-MS spectrogram see Figure 4 ) sample 500mg, dissolved in 3ml tetrahydrofuran, added 0.05g lanthanum nitrate and mixed thoroughly, slowly added dropwise 0.25ml acetic anhydride, reacted at -78°C (dry ice-ethanol) for 3 hours, after heating to 0°C, added saturated NaHCO3 The reaction was quenched with 2ml of the solution, and then 200mg of mCPBA was added. After 3 hours, TLC detected that the reaction was complete. After the reaction, the feed liquid was extracted with ethyl acetate, dried over anhydrous sodium sulfate, and 0.47g white solid was obtained by rotary evaporation in vacuo, which was purified by silica gel column chromatography to obtain 0.39g cephalomannine (content 90%, yield 78.5 %, LC-MS spectrum see Figure 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com