Environment-friendly acid leaching-extraction process in waste lithium battery recovery
A waste lithium battery and acid leaching technology, which is applied in the direction of improving process efficiency and removing solid waste, can solve problems such as large waste water, achieve the effects of reducing alkali consumption, reducing waste water discharge, and reducing overall costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
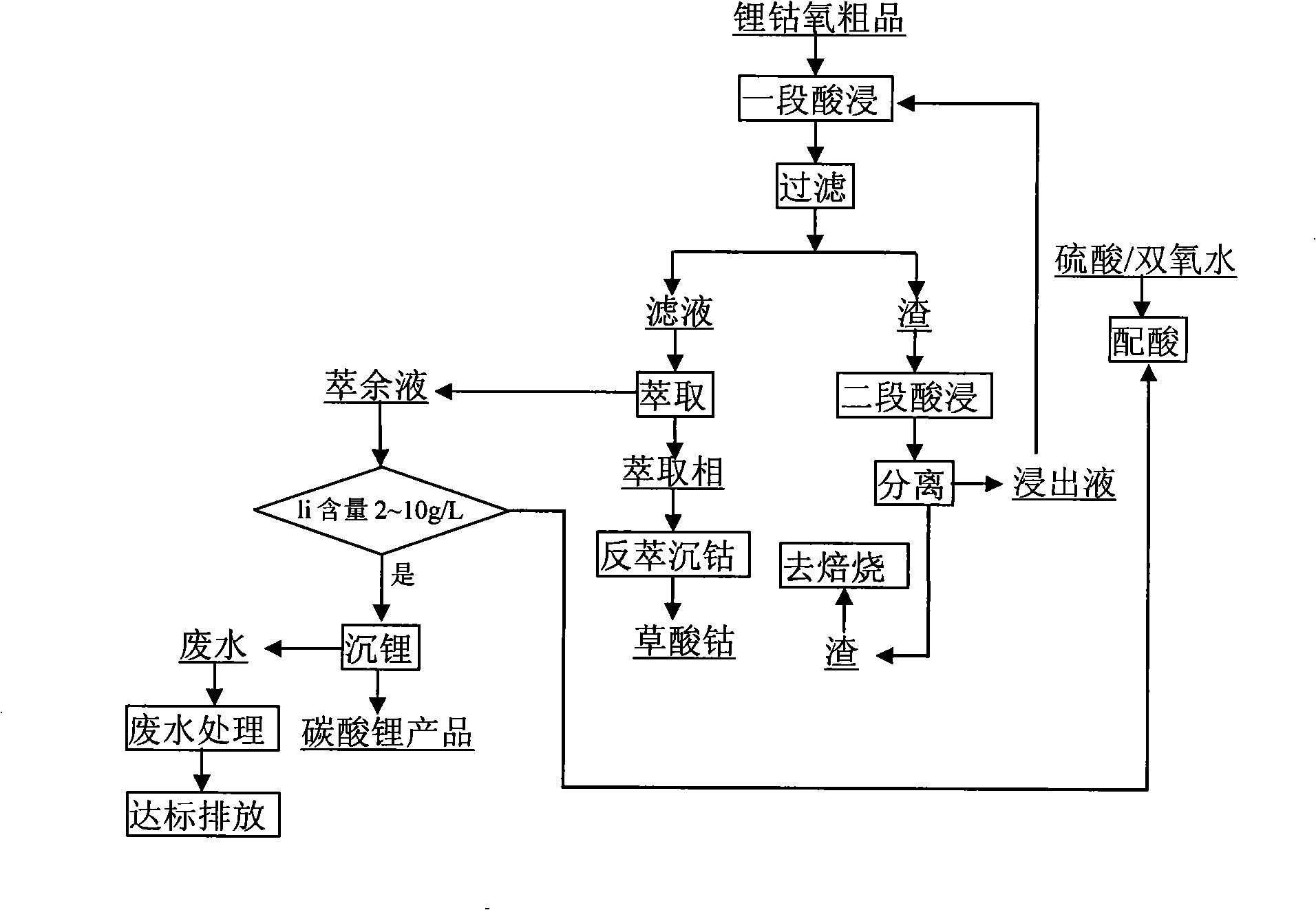
[0009] Embodiment 1: as attached figure 1 In the process shown, 10 kg of waste mobile phone lithium batteries are physically disassembled and shelled to obtain 5.21 kg of crude lithium cobalt oxide (lithium cobaltate and carbon powder), which are stirred and leached in two stages at 80 ° C. The leaching solution is sulfuric acid / Hydrogen peroxide mixed solution (sulfuric acid volume concentration 20%, hydrogen peroxide volume concentration 20%), volume is 6L, two-stage countercurrent leaching, final pH of leaching solution is 4.1, temperature 80 ℃. The leaching solution uses P507 extractant (25% P507 + 25% TBP + 50% sulfonated kerosene, compared to 1) for secondary extraction of cobalt in the solution, back extraction of cobalt with oxalic acid, washing and drying. The raffinate is reconfigured into a leaching solution with a sulfuric acid volume concentration of 20% and a hydrogen peroxide volume concentration of 20%, and returns to the leaching section for reuse, wherein th...
Embodiment 2
[0010] Embodiment 2: adopt this process, under the stirring condition of 85 ℃, use 70L leaching solution two-stage countercurrent leaching lithium cobalt oxygen crude product (lithium cobaltate and carbon powder), two-stage extraction, leaching solution is sulfuric acid / hydrogen peroxide mixed solution (sulfuric acid volume Concentration 15%, hydrogen peroxide volume concentration is 15%), final pH of leachate is 4.5. Add acid and hydrogen peroxide to the raffinate until the volume concentration of sulfuric acid is 15%, and the volume concentration of hydrogen peroxide is 15%, and return to the leaching section for reuse, wherein the consumed water is replenished with washing water during filtration. The raffinate repeats the above cycle 10 times. 70L leaching solution leached 43kg of lithium cobalt oxygen crude product altogether, obtained 37.1kg cobalt oxalate (CoC 2 o 4 .2H 2 O) product, product cobalt content 30.96%, cobalt recovery rate 97.7%. The composition of the e...
Embodiment 3
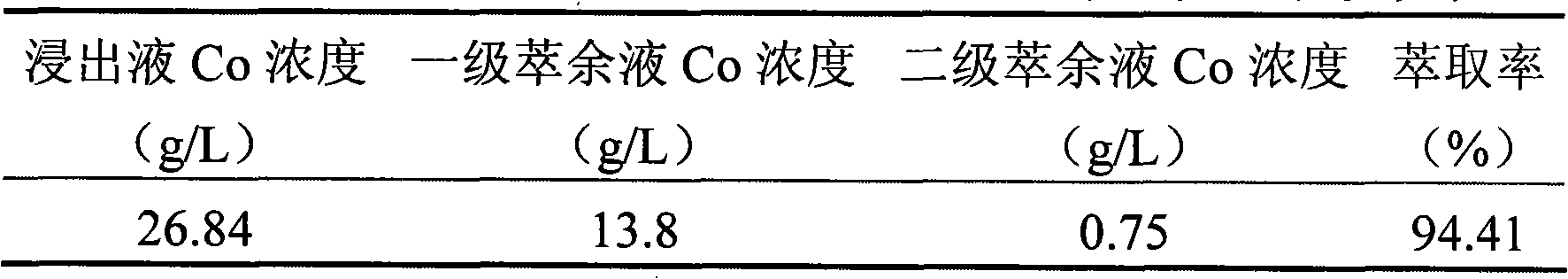
[0013] Embodiment 3: Use this process to process 1 ton of waste lithium-ion batteries per day, two-stage countercurrent leaching, two-stage extraction, the leachate is a sulfuric acid / hydrogen peroxide mixed solution (the concentration of sulfuric acid is 18%, the concentration of hydrogen peroxide is 20%), and the volume is 7m 3 , the temperature is 80-85°C, the pH of the final leaching solution is 4-4.5, and the average leaching rate is 89%. The extracted organic phase composition is: 25% P507+25% TBP+50% sulfonated kerosene, TBP is a synergistic extractant, the total extraction rate is 94.57%, the overall recovery rate of cobalt is 99.1%, and the cobalt oxalate product meets the BH-JZ-2328 standard Require. The raffinate was recycled 10 times. After 2 months, the implementation effect is as follows: the wastewater discharge of leachate has changed from 2000m 3 The monthly discharge of raffinate is reduced to 240m 3 The monthly discharge of raffinate reduces the discharge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com