Enoxacin freeze-dried powder needle composing prescription and preparation technique
A preparation technology of enoxacin, which is applied in the field of medicine, can solve the problems of light and high temperature sensitivity of liquid medicine, low bioavailability of solid preparations, poor solubility of enoxacin, etc., and achieve short liquid preparation time and convenient transportation and clinical application, and the effect of improving the safety of clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] prescription:
[0025] Enoxacin 100g
[0026] Mannitol 50g
[0027] pH3.6 acetic acid-sodium acetate buffer 300g
[0028]
[0029] A total of 1000 bottles were produced
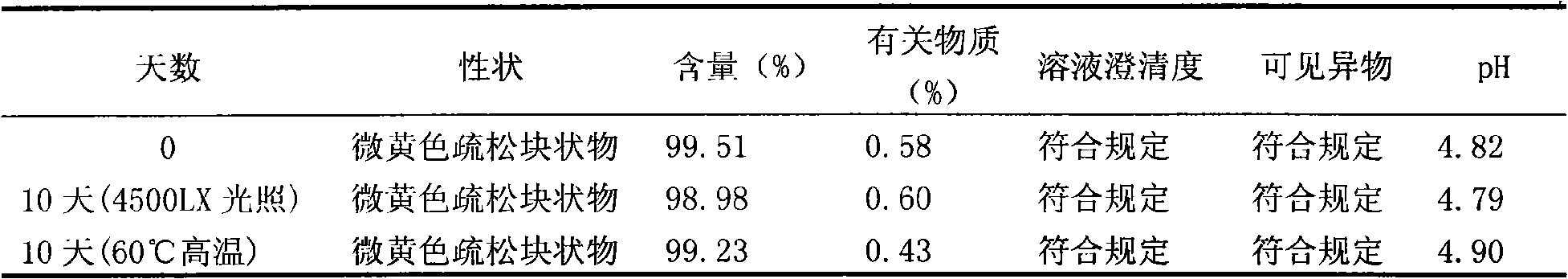
[0030] Preparation process: Take 100g of enoxacin and 50g of mannitol in a liquid mixing tank, add 500ml of water for injection; take 300g of acetic acid-sodium acetate buffer solution with pH 3.6 and slowly add it, stir until completely dissolved; add water for injection to 1000ml, stir well. Add 1g of medicinal charcoal and stir for 30 minutes, then use a titanium filter rod and then use a 0.22μm microporous membrane filter to filter into a sterile room. The detected pH value is 4.8, and 1ml of each bottle is divided into antibiotic glass bottles, and the stopper is half-pressed. Put it in a freeze-drying box, quickly cool down to -50°C, and prefreeze for 2 hours; turn on the vacuum pump, control the temperature of the heat transfer oil to -20°C, and continu...
Embodiment 2
[0032] prescription:
[0033] Enoxacin 200g
[0034] Mannitol 50g
[0035] pH2.0 Phosphate Buffer 150g
[0036]
[0037] A total of 1000 bottles were produced
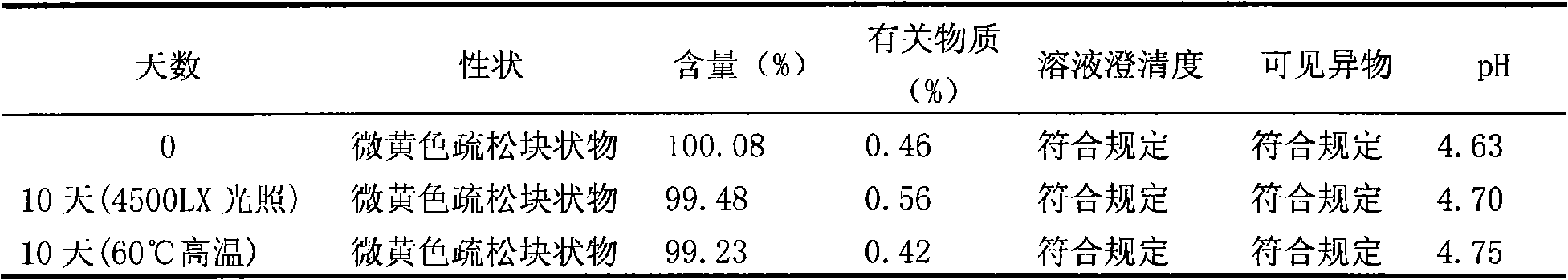
[0038] Preparation process: Take 200g of enoxacin and 50g of mannitol in a liquid preparation tank, add 1000ml of water for injection; take 150g of pH 2.0 phosphate buffer solution, add slowly, stir until completely dissolved; add water for injection to 2000ml, Stir well. Add 2g of medicinal charcoal and stir for 30 minutes, then use a titanium filter rod and then use a 0.22μm microporous filter membrane to filter it into a sterile room. The detected pH value is 4.6, and 2ml of each bottle is divided into antibiotic glass bottles, and the stopper is half-pressed. Put it in a freeze-drying box, quickly cool down to -50°C, and pre-freeze for 3 hours; turn on the vacuum pump, control the temperature of the heat transfer oil to -20°C, and continuously vacuumize for 15 hours; gradua...
Embodiment 3
[0040] prescription:
[0041] Enoxacin 200g
[0042] Mannitol 50g
[0043] Lactic acid 50g
[0044]
[0045] A total of 1000 bottles were produced
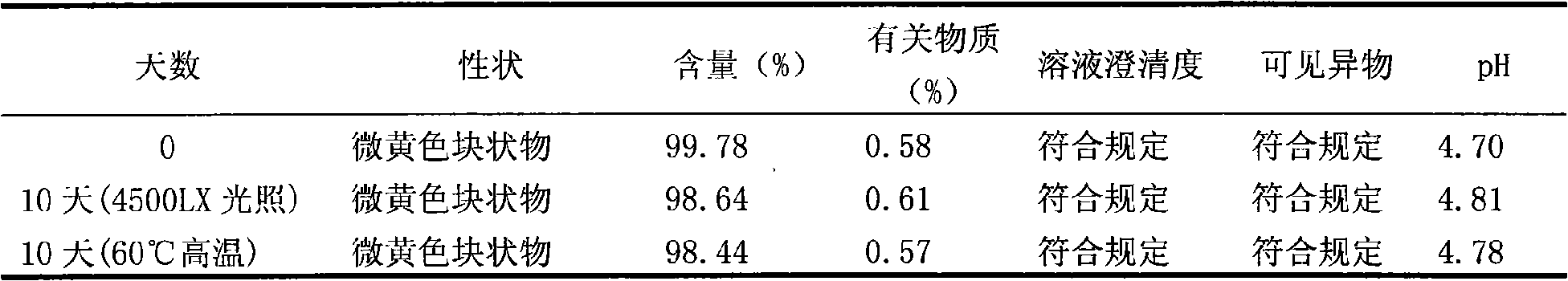
[0046] Preparation process: Take 200g of enoxacin and 50g of mannitol in a liquid mixing tank, add 1000ml of water for injection; slowly add 50g of lactic acid, stir to completely dissolve; add water for injection to 2000ml, and stir evenly. Add 2g of medicinal charcoal and stir for 30 minutes, then use a titanium filter rod and then use a 0.22μm microporous filter membrane to filter it into a sterile room. The pH value was detected to be 4.7, and 2ml of each bottle was divided into antibiotic glass bottles, and the stopper was half-pressed. Put it in a freeze-drying box, quickly cool down to -50°C, and pre-freeze for 3 hours; turn on the vacuum pump, control the temperature of the heat transfer oil to -20°C, and continuously vacuumize for 16 hours; gradually raise the temperature to 25°C, and conti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com