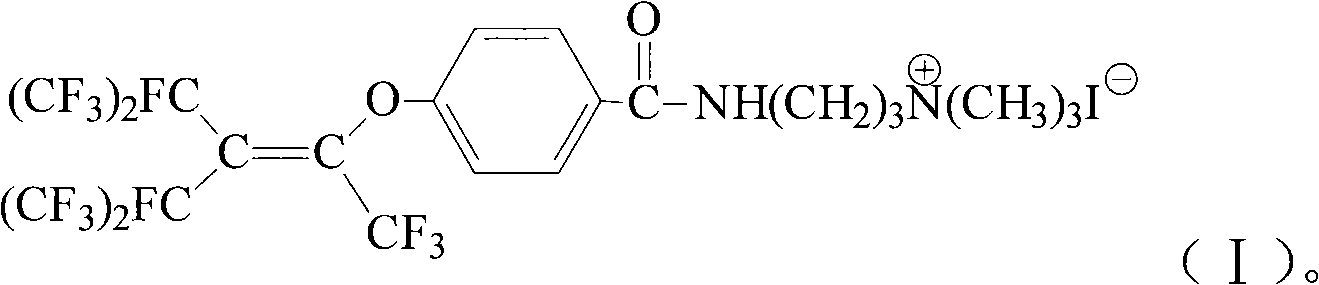
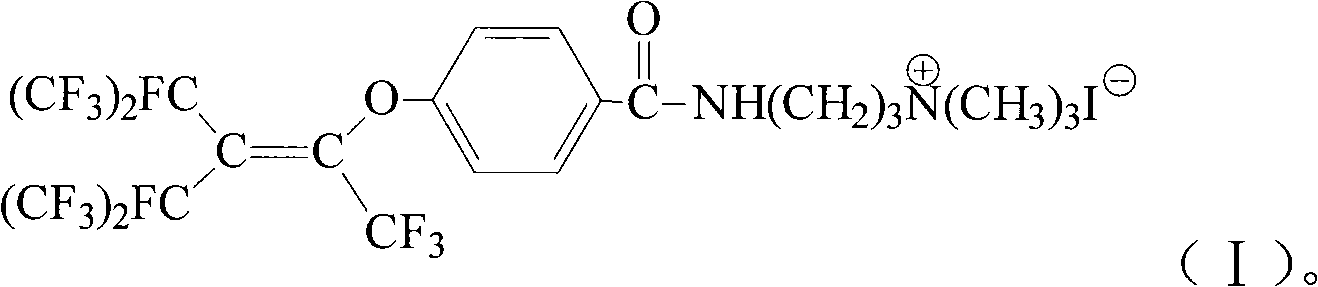
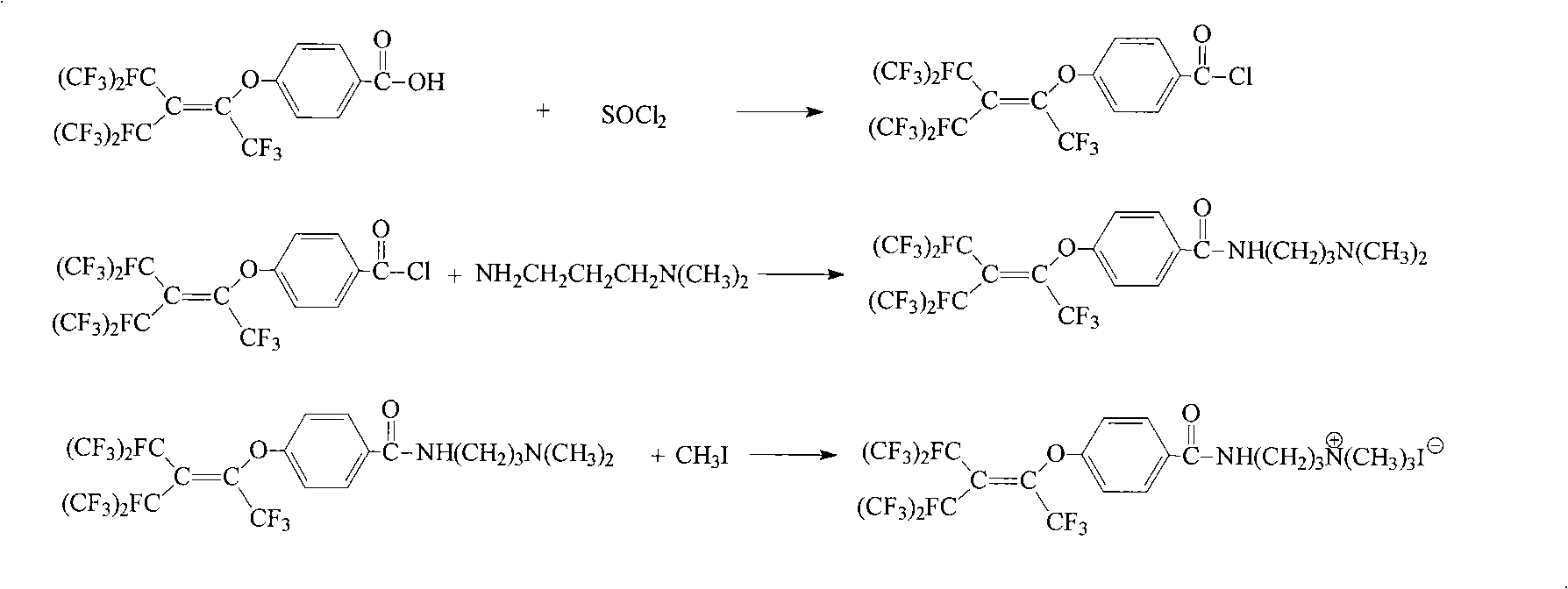
Cation fluorine surfactant and preparation method thereof
A fluorosurfactant and cation technology, applied in the field of cationic fluorosurfactant and its preparation, to achieve the effects of low surface tension, simple synthesis method and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 50mL trichloroethane, 28.4g (0.05mol) p-perfluorononenyloxybenzoic acid (Lianyungang Taizhuo New Material Co., Ltd.) into a 250mL four-necked flask, stir, heat to 40℃, and slowly add 7.14 g(0.06mol) thionyl chloride, continue to stir for 3h. Raise the temperature to 76°C, keep the temperature and stir until no gas escapes. Cool to 50℃, slowly add 5.1g (0.05mol) N,N-dimethylpropanediamine in trichloroethane (10mL) solution, stir for 0.5h, add 5.3g (0.05mol) sodium carbonate powder, stir Reaction for 3h. Cool to room temperature, add 50 mL of water and stir, stand still for layering, separate to obtain an organic phase, and distill under reduced pressure to remove trichloroethane to obtain a solid. Add this solid substance and 30 mL acetonitrile to a 100 mL four-neck flask, and heat and stir until the solid is dissolved. The temperature was continued to rise to 60°C, and a solution of 7.1g (0.05mol) methyl iodide in acetonitrile (10 mL) was slowly added dropwise, and the ...
Embodiment 2
[0033] Add 50 mL of trichloroethane and 28.4 g of p-perfluorononenyloxy benzoic acid to a 250 mL four-necked flask, stir, heat to 30° C., slowly add 8.9 g (0.075 mol) thionyl chloride dropwise, and continue stirring for 4 hours. Raise the temperature to 76°C, keep the temperature and stir until no gas escapes. Cool to 60°C, slowly add 6.1g (0.06mol) N,N-dimethylpropanediamine in trichloroethane (10 mL) solution, stir for 0.5h, add 8.0g (0.075mol) sodium carbonate powder, The reaction was stirred for 4h. Cool to room temperature, add 50 mL of water and stir, stand still for layering, separate the organic phase, and distill under reduced pressure to remove the trichloroethane to obtain a solid. The solid matter and 30 mL of ethyl acetate were added to a 100 mL four-neck flask, and heated and stirred until the solid was dissolved. The temperature was continued to rise to 70°C, and a solution of 8.5g (0.06mol) methyl iodide in ethyl acetate (10 mL) was slowly added dropwise, and stirr...
Embodiment 3
[0035]Add 50mL cyclohexanone and 28.4g p-perfluorononenyloxybenzoic acid to a 250mL four-necked flask, stir, heat up to 50°C, slowly add 9.5g (0.08mol) thionyl chloride dropwise, and continue stirring for 6h. Raise the temperature to 76°C, keep the temperature and stir until no gas escapes. Cool to 70°C, slowly add 7.7g (0.075mol) N,N-dimethylpropanediamine in cyclohexanone (10mL) solution dropwise, stir for 0.5h, add 8.1g (0.08mol) triethylamine, stir to react 5h. Cool to room temperature, add 50 mL of water and stir, stand still for layering, separate to obtain an organic phase, and distill under reduced pressure to remove cyclohexanone to obtain a solid. Add the solid matter and 30 mL of N,N-dimethylformamide to a 100 mL four-neck flask, and heat and stir until the solid is dissolved. The temperature was continued to rise to 80°C, and 11.3g (0.08mol) of methyl iodide in N,N-dimethylformamide (10 mL) was slowly added dropwise, and stirred at 80°C for 5h. The solvent was distille...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com