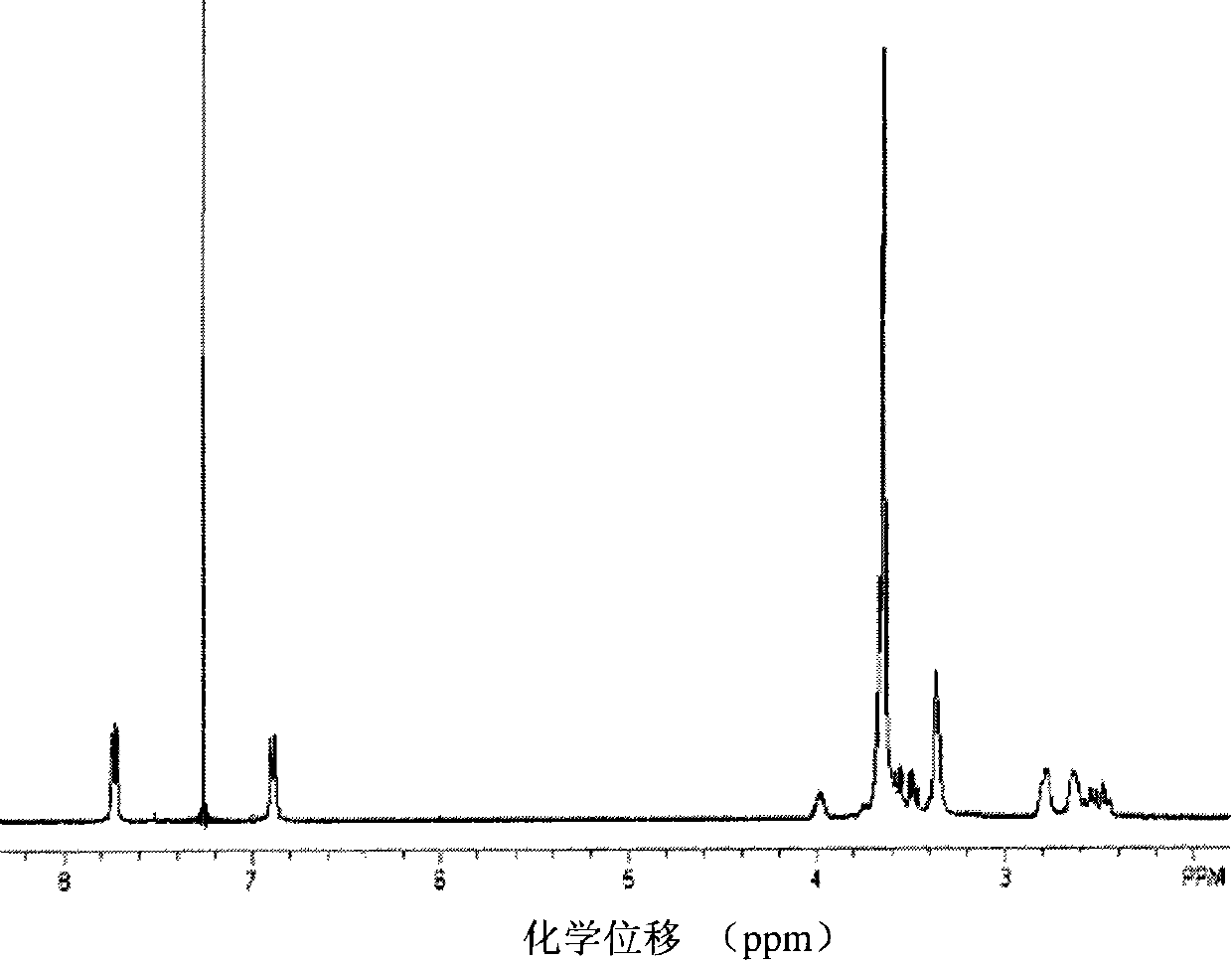
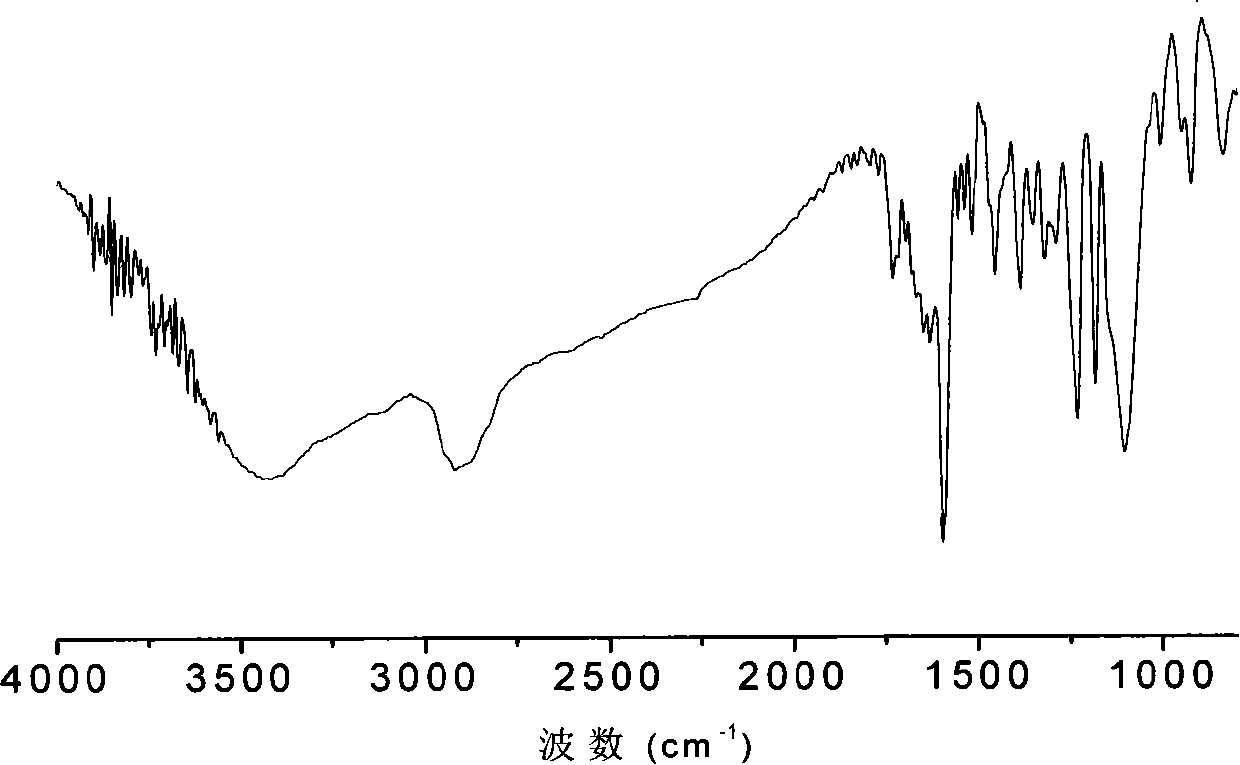
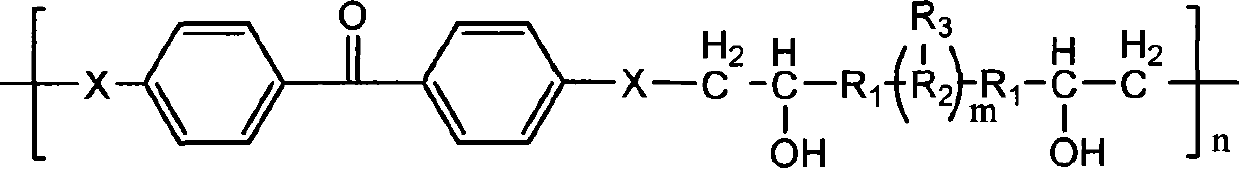
Method for preparing polymer type michler's ketone photoinitiator
A photoinitiator and polymer-based technology, applied in the field of photochemistry, can solve the problems of no significant improvement in initiation performance, limited application fields, and reduced initiation efficiency, and achieve the effects of improving photoinitiation efficiency, broad application prospects, and improving performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] a) In a 100ml three-neck flask with magnetic stirring and nitrogen gas, add 0.02mol (4.36g) of 4,4'-difluorobenzophenone, 0.2mol (17.23g) of piperazine, 0.05mol (6.95g) in anhydrous potassium carbonate and 25ml dimethyl sulfoxide, reacted at 160°C in the dark for 50 hours, cooled to room temperature, then poured into 10 times the volume of water to obtain a yellow precipitate, filtered, and put the filter cake at 80°C Vacuum-dried to obtain 2.27 g of a small molecule photoinitiator 4,4'-dipiperazine benzophenone with a yield of 66%.
[0031] b) Add 2mmol (1.0492g) of polyoxyethylene glycidyl ether to 10ml of N,N-dimethylformamide to dissolve completely, and add 1.9mmol (0.6634g) of 4,4'-dipiperazine diphenyl under nitrogen protection Methanone was heated to 75° C. for 8 hours, cooled to room temperature, then poured into 10 times the volume of anhydrous ether, filtered, and the obtained solid was vacuum-dried to obtain 1.30 g of a polymer-type michroketone photoinitiato...
Embodiment 2
[0033] a) In a 100ml three-neck flask with magnetic stirring and nitrogen gas, add 0.02mol (4.36g) of 4,4'-difluorobenzophenone, 0.2mol (17.23g) of piperazine, 0.05mol (6.95g) in anhydrous potassium carbonate and 25ml dimethyl sulfoxide, reacted at 160°C in the dark for 50 hours, cooled to room temperature, then poured into 10 times the volume of water to obtain a yellow precipitate, filtered, and put the filter cake at 80°C Vacuum-dried to obtain 2.27 g of a small molecule photoinitiator 4,4'-dipiperazine benzophenone with a yield of 66%.
[0034] b) Dissolve 2mmol (0.4084g) glycerol diglycidyl ether completely in 10ml ethanol, then add 1.9mmol (0.6659g) 4,4'-dipiperazine benzophenone under nitrogen protection, and heat up to 80 ℃, reacted for 5 hours, cooled to room temperature, and then poured into 10 times the volume of anhydrous ether, poured out the liquid after the precipitation was complete, and dried in vacuum. Obtain 0.91 g of high-molecular-weight michketone photoi...
Embodiment 3
[0036] a) In a 100ml three-neck flask with magnetic stirring and nitrogen gas, add 0.02mol (4.36g) of 4,4'-difluorobenzophenone, 0.2mol (17.23g) of piperazine, 0.05mol (6.95g) in anhydrous potassium carbonate and 25ml dimethyl sulfoxide, reacted at 160°C in the dark for 50 hours, cooled to room temperature, then poured into 10 times the volume of water to obtain a yellow precipitate, filtered, and put the filter cake at 80°C Vacuum-dried to obtain 2.27 g of a small molecule photoinitiator 4,4'-dipiperazine benzophenone with a yield of 66%.
[0037]b) Add 2mmol (1.28g) polypropylene oxide glycidyl ether to 10ml ethanol to dissolve completely, add 1.9mmol (0.6634g) 4,4'-dipiperazine benzophenone under nitrogen protection, and heat up to 75°C , reacted for 6 hours, cooled to room temperature, and then poured into 10 times the volume of anhydrous ether to obtain a yellow viscous liquid, which was dried in vacuum to obtain 1.55 g of macromolecular micone photoinitiator, with a yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com