Method for collecting nickel and cobalt from laterite-nickel ore lixivium
A technology for separation and enrichment of laterite nickel ore, applied in the field of non-ferrous hydrometallurgy, can solve the problems of complex treatment process, large impurity content, large nickel loss, etc., and achieve the effects of simple process path, high extraction rate and low operating cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
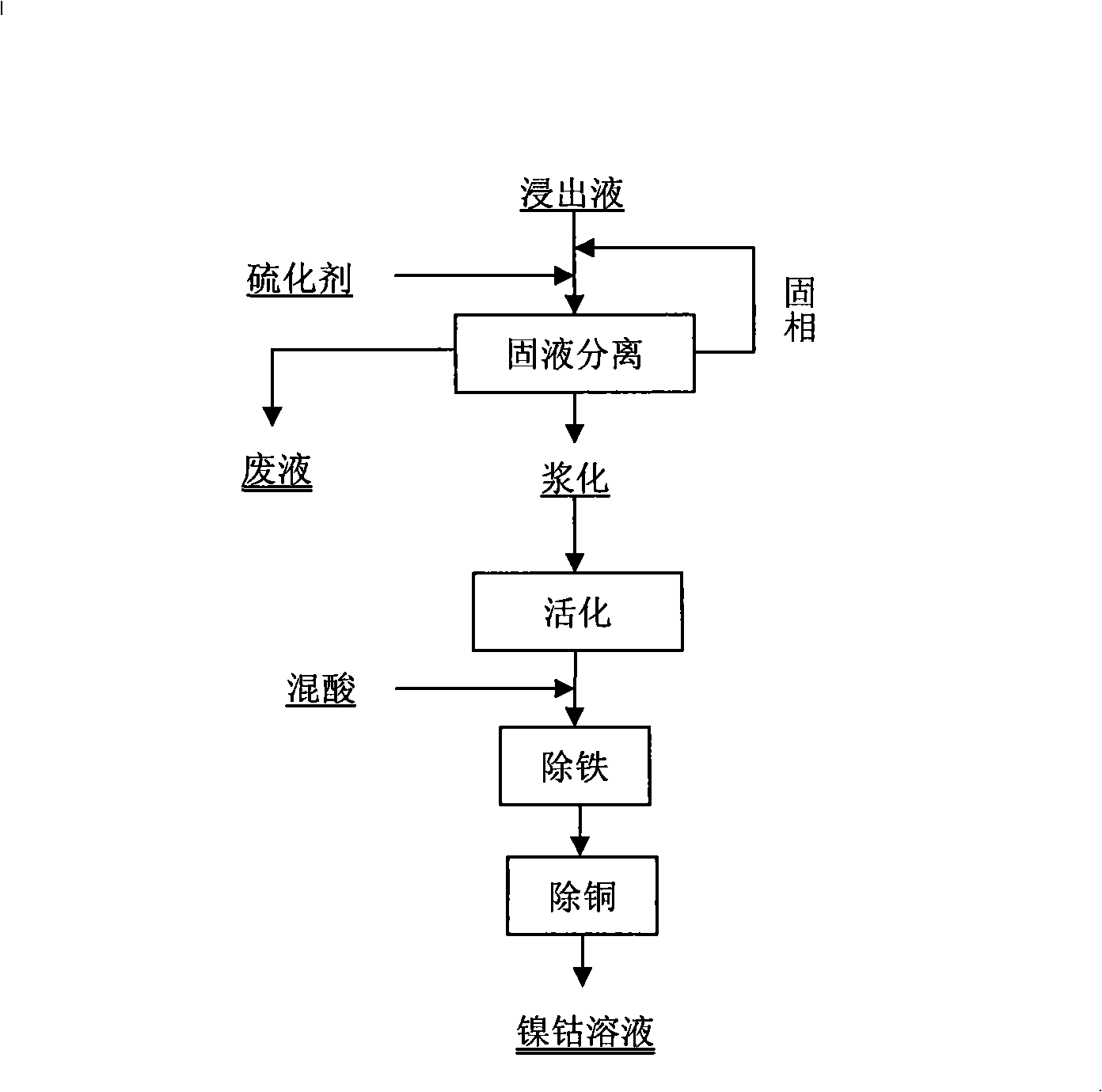
[0021] A. Put 25 liters of laterite ore leaching solution into a 50 liter reaction vessel, in the leaching solution: Ni 6g / L, Fe 33g / L, Cu 1g / L, Co 0.98g / L, Mg 3.3g / L, Zn 0.57g / L. Ammonium sulfide is dropped into 1.2 times the stoichiometric ratio of the metal equivalent in the leaching solution, and the pH value rises to 3.0;
[0022] B. After the solid-liquid separation of the precipitate, wash the precipitate with a new leaching solution for 10 minutes, and the pH rises to 1;
[0023] C. After the sulfide precipitation after washing and soaking, ferrous sulfide accounts for 5.6% of the total sulfide precipitation mass after solid-liquid separation, add water to it to form a slurry with a solid-liquid ratio of 1:3, and activate it by mechanical stirring in a stirring tank for 15 minutes , stirring speed 280rpm;
[0024] D, the slurry after activation is added sulfuric acid nitric acid mixed acid (sulfuric acid: the volume ratio of nitric acid is 90: 10) solution by 1.5 ti...
Embodiment 2
[0028] A, drop 50 liters of leachate containing Ni 4.4g / L, Fe 38g / L, Cu 0.78g / L, Co 0.88g / L, Mg 2.3g / L, Zn 0.69g / L laterite sulfuric acid system into 100 liters of In the reaction kettle, sodium sulfide is added according to 1.3 times the stoichiometric ratio of the metal amount, and the pH value rises to 3.46.
[0029] B. Fully wash the sulfide precipitate after the solid-liquid separation in the previous step with the new leachate for 15 minutes, raise the pH to about 1 and perform solid-liquid separation;
[0030] C, the solid phase after the separation of the previous step is slurried by a solid-to-liquid ratio of 1: 3, the slurry is mechanically stirred and activated for 20 minutes, the stirring speed is 300rpm, and ferrous sulfide in the solid phase accounts for 10.4% of the total precipitated mass of sulfide;
[0031] D, add sulfuric acid nitric acid mixed acid (sulfuric acid: the volume ratio of nitric acid is 90: 10) solution to the slurry after activation by relative...
Embodiment 3
[0035] A. Put 25 liters of laterite ore sulfuric acid system leaching solution into a 50 liter reaction vessel, in the leaching solution: Ni 6g / L, Fe 36g / L, Cu 1g / L, Co 0.68g / L, Mg 4.3g / L, Zn 0.55g / L. Ammonium sulfide is added according to 1.5 times the amount of metal in the leaching solution, and the pH value rises to 3.5;
[0036] B. After the solid-liquid separation of the precipitate, the precipitate is fully washed with the new leachate for 20 minutes, and the pH rises to 1;
[0037] C, after the sulfide precipitation after washing and soaking, ferrous sulfide accounts for 5.9% of the total sulfide precipitation mass after solid-liquid separation, it is added with water to form a slurry with a solid-to-liquid ratio of 1: 3, and is activated by mechanical stirring in a stirring tank for 30 Minutes, stirring speed 320rpm;
[0038] D. Add sulfuric acid and nitric acid mixed acid to the activated slurry according to 1.5 times the metal equivalent stoichiometric ratio in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com