Dengue virus IgG antibody ELISA diagnostic kit
A technology for dengue virus and immunodiagnosis, which is applied in the field of dengue virus antibody enzyme-linked immunoassay diagnostic kits, can solve the problems of high price, difficult to popularize, delayed diagnosis and the like, and achieves the prospect of wide popularization and application, the method is simple and the sensitivity is high Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
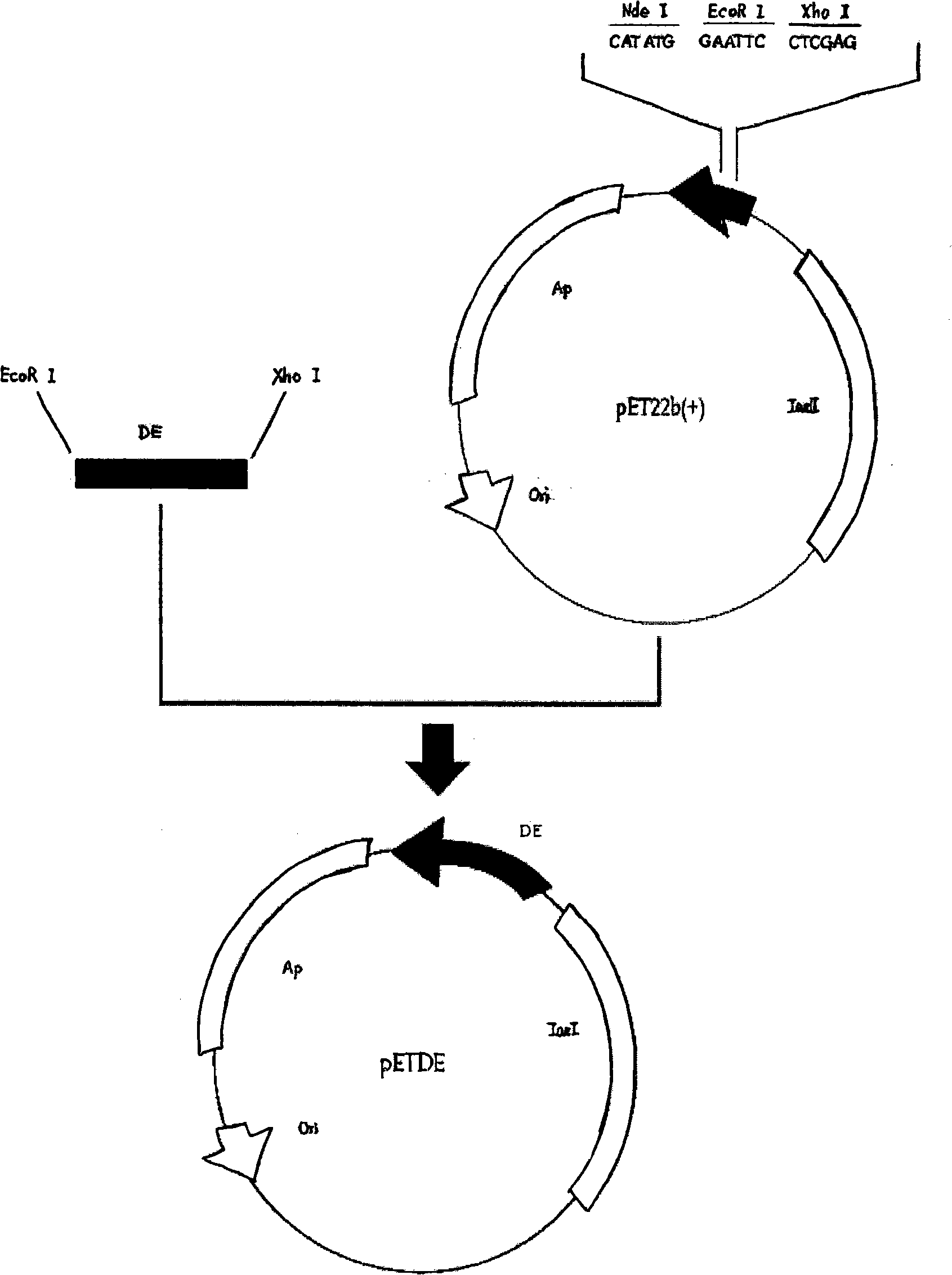
[0020] Example 1: Cloning, expression and purification of dengue antigen
[0021] (1) Materials
[0022] 1. Standard strains: Dengue type 1 (Hawaii strain), referred to as D1; Dengue type 2 (NGC strain), referred to as D2; Dengue type 3 (H87 strain), referred to as D3; Dengue type 4 (H241 strain) , referred to as D4; purchased from the National Institute for the Control of Pharmaceutical and Biological Products, and preserved by the Guangdong Provincial Center for Disease Control and Prevention.
[0023] 2. Plasmid: The cloning vector BM13 plasmid was purchased from TaKaRa Company, and the expression plasmid pET22b(+) was a product of Novagen. Preserved by the Microbiology Laboratory of the Guangdong Provincial Center for Disease Control and Prevention.
[0024] 3. Bacterial strains: E. coli TOP10F′ for recipient and E. coli BL21 Star for expression TM (DE3) was purchased from Invitrogen and preserved by the Institute of Microbiology, Guangdong Provincial Center for Disea...
Embodiment 2
[0102] Embodiment two: the mensuration of recombinant antigen to dengue virus IgG antibody reactivity
[0103] The purified extracted protein was serologically tested by indirect ELISA to determine its reactivity to dengue virus antibody-positive sera.
[0104] The antigen obtained in Example 1 was diluted 1:500, 1:1000, 1:2000, coated on an ELISA plate, and reacted with dengue positive serum to determine the concentration of the coated antigen. The immune activity of dengue antigen is 1:1000.
[0105] The recombinant antigen was coated with selected concentration, and the serum IgG antibody of dengue fever patients was detected by indirect method ELISA, and the reactivity of the recombinant antigen to dengue virus IgG antibody was determined.
[0106] 1. Reactivity of recombinant antigens to serum samples from the 2004 Zhongshan dengue fever outbreak
[0107] Type 1-4 E protein recombinant antigens were coated at a selected concentration, and the IgG antibodies in the serum...
Embodiment 3
[0121] Embodiment three: the production technology of dengue virus IgG antibody ELISA kit according to the present invention
[0122] (1) Materials
[0123] 1. Dengue antigen: see Example 1 for preparation. When the antigen is coated at a ratio of 1:1000, the positive coincidence rate to the in-factory reference product reaches 98%, and the specificity reaches 97%.
[0124] 2. Goat anti-human IgG-HRP: product of Shenzhen Ruisang Company.
[0125] 3.TMB: German Bollinger product.
[0126] 4. Positive control serum: the positive control serum is made by adding the positive serum of a certain concentration in the PBS containing 20% goat serum and 0.02% sodium azide 10mM pH7.4, and the A value of the positive control should be Greater than 1.5.
[0127] 5. Negative control serum: Negative control serum was prepared by adding 5% of the mixed negative serum to 10 mM pH 7.4 PBS containing 20% goat serum and 0.02% sodium azide. The A value of the negative control should be le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com