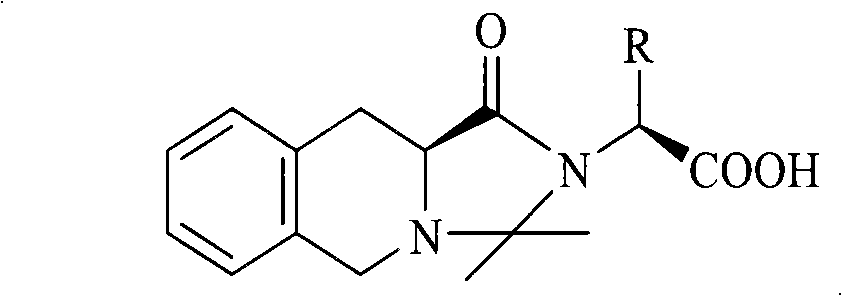
Isoquinolinium compounds with antithrombotic activity, preparation method and application thereof
A technology of antithrombotic drugs and compounds, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
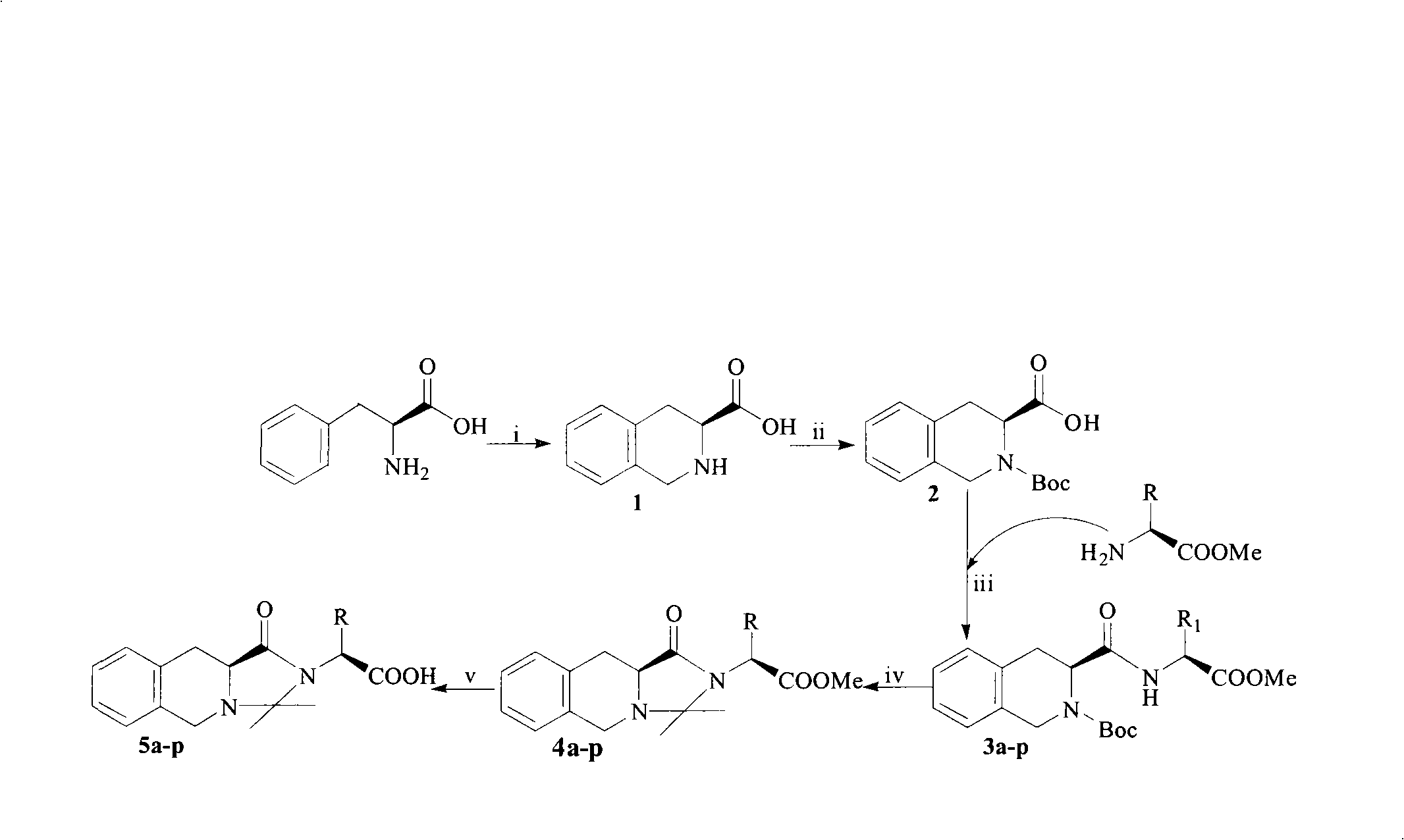
[0023] Example 13 Preparation of S-1,2,3,4-tetrahydroisoline-3-carboxylic acid hydrochloride (1)
[0024] 1.65 g (10 mmol) of L-phenylalanine was dissolved in 90 mL of concentrated hydrochloric acid, and 35 mL of formaldehyde solution with a concentration of 35% was added to the obtained solution. The reaction mixture was reacted at 90°C for 8-9 hours, and the disappearance of L-phenylalanine was monitored by thin layer chromatography to terminate the reaction. The reaction was cooled to room temperature, and colorless crystals were precipitated, which was filtered, and the filter cake was washed with acetone to obtain 1.92 g (95.4%) of the title compound as a colorless solid. ESI / MS: 178[M+H]+;
Embodiment 23
[0025]Example 23 Preparation of S-N-Boc-1,2,3,4-tetrahydroisoline-3-carboxylic acid (2)
[0026] 4.0 g (18.7 mmol) of 3S-1,2,3,4-tetrahydroisoline-3-carboxylic acid hydrochloride (1) were dissolved in 40 ml of DMF. To this suspension was added 5.2 g (23.9 mmol) of Boc with stirring in an ice bath 2 O. Triethylamine was added to adjust the pH of the reaction mixture to 10, the reaction mixture was stirred at room temperature for 48 hours, monitored by thin layer chromatography until the disappearance of the raw materials, and the reaction was terminated. Pour the reaction solution into a watch glass and blow it under a fan for about 24 hours to dry. The dried oil was dissolved in 200 mL of ethyl acetate, and then placed in a 250 mL separatory funnel, and KHSO was added to the solution. 4 (5%) aqueous solution (20 mL x 3). Separate the combined ethyl acetate layers, add anhydrous sodium sulfate to a 250 mL conical flask, dry for 0.5 h, and filter at normal pressure. The fil...
Embodiment 33
[0027] Example 33 Preparation of S-N-Boc-1,2,3,4-tetrahydroisoline-3-formyl-L-leucine methyl ester (3a)
[0028] Under ice bath cooling, 2.00 g (7.22 mmol) of 3S-N-Boc-1,2,3,4-tetrahydroisoline-3-carboxylic acid, 0.97 g (7.19 mmol) of N- Hydroxybenzotriazole (HOBt) and 1.37g (7.55mmol) of L-leucine methyl ester hydrochloride and 70ml of anhydrous tetrahydrofuran (THF) were added dropwise to 1.70g (8.25mmol) of dicyclohexylcarbonyldiimide. (DCC) and 5 mL of anhydrous THF. The resulting solution was adjusted to pH 8 by dropwise addition of a solution of NMM and THF. The reaction mixture was stirred at 0°C for 2 hours, filtered with suction, and the filtrate was concentrated to dryness under reduced pressure using a rotary evaporator. The obtained residue was dissolved in 150 mL of ethyl acetate, placed in a 250 mL separatory funnel, washed three times with saturated aqueous sodium bicarbonate solution (30 mL each time), washed three times with saturated aqueous sodium chloride...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com