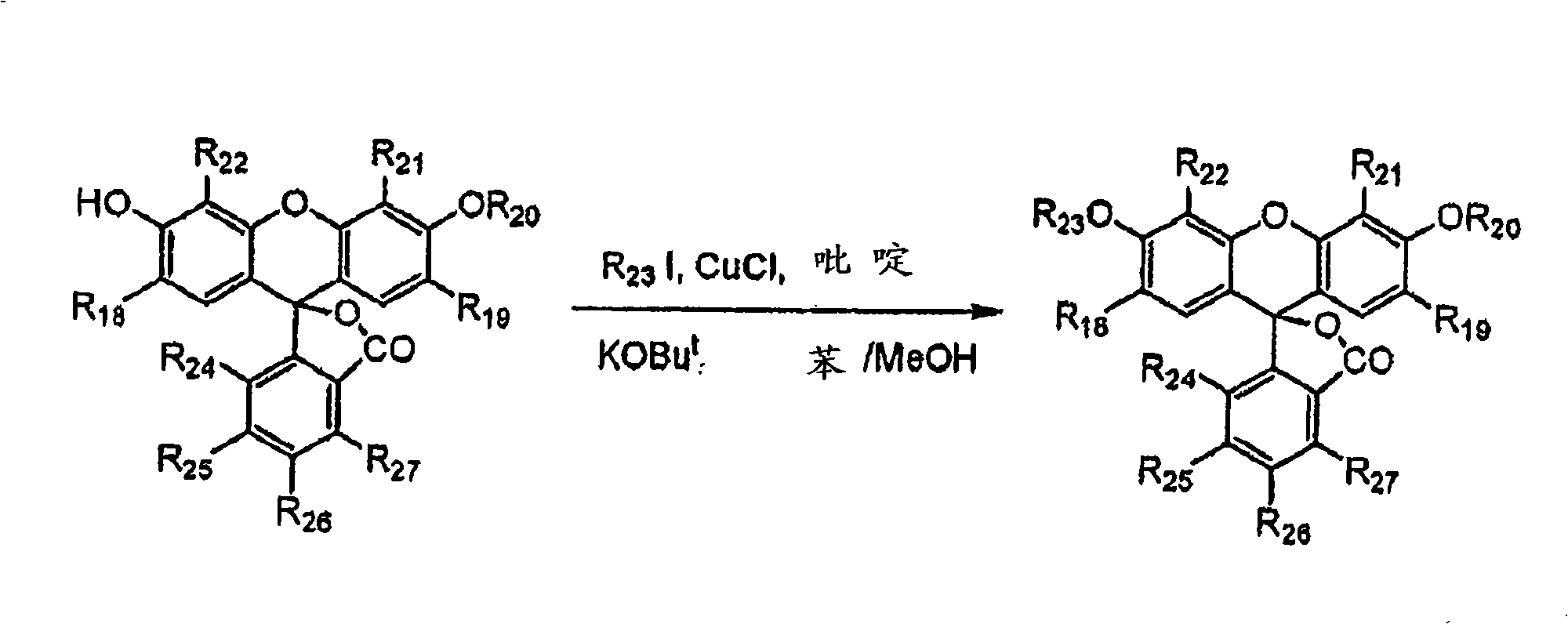Reagents for highly specific detection of peroxynitrite
一种亚硝酸根、专一的技术,应用在过亚硝酸根的检测领域,能够解决灵敏度低、耗时、烦琐等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
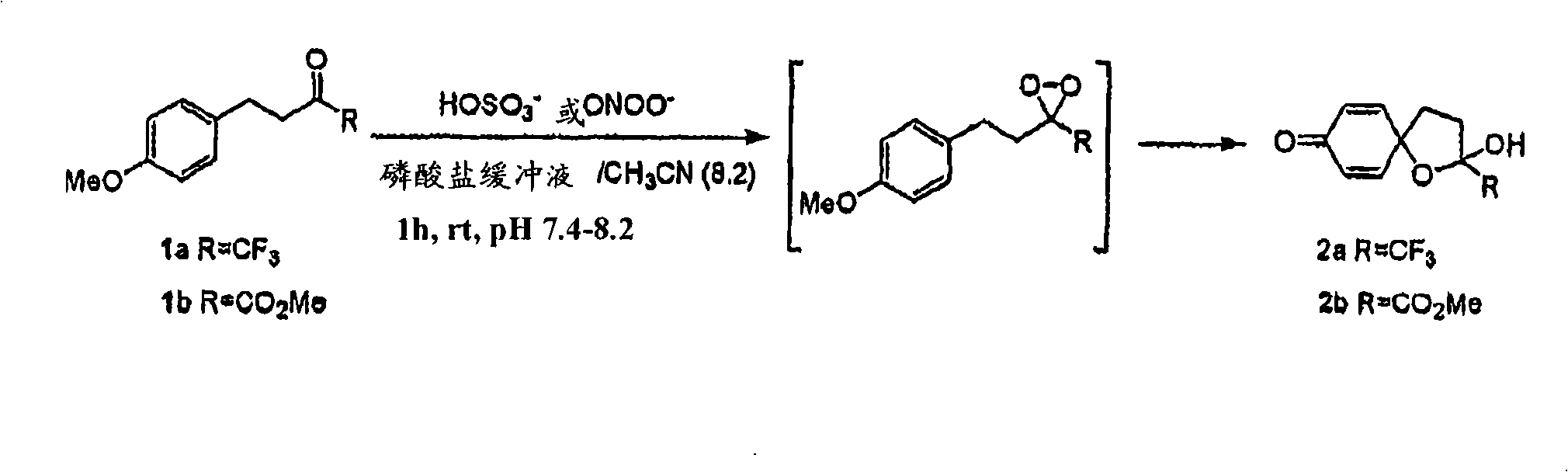
[0046] As noted above, the present invention provides compounds that react exclusively with pernitrite and do not react with other reactive oxygen intermediates and reactive nitrogen intermediates. The compound has the following general formula (I):
[0047]
[0048] in:
[0049] R 1 for OR 1 ’ or NR 2 'R 3 ’, where R 1 ’, R 2 ’ and R 3 'independently hydrogen or selected from alkyl, alkenyl, alkynyl, alkoxyalkyl, alkanoyl, alkenoyl, alkynoyl, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, alkaryl , aralkyl, aroyl or polyether groups;
[0050] R 2 , R 3 , R 4 and R 5 independently hydrogen or a group selected from halogen, alkyl, alkoxy, alkyloxy, polyether; R 2 and R 3 together form a 5, 6 or 7 membered ring selected from aryl, heterocyclic, heteroaryl or heteroaromatic; or R 4 and R 5 together form a 5, 6 or 7 membered ring selected from aryl, heterocycle, heteroaryl or heteroaryl;
[0051] R 6 is an electron-withdrawing group selected from CF 3 , haloge...
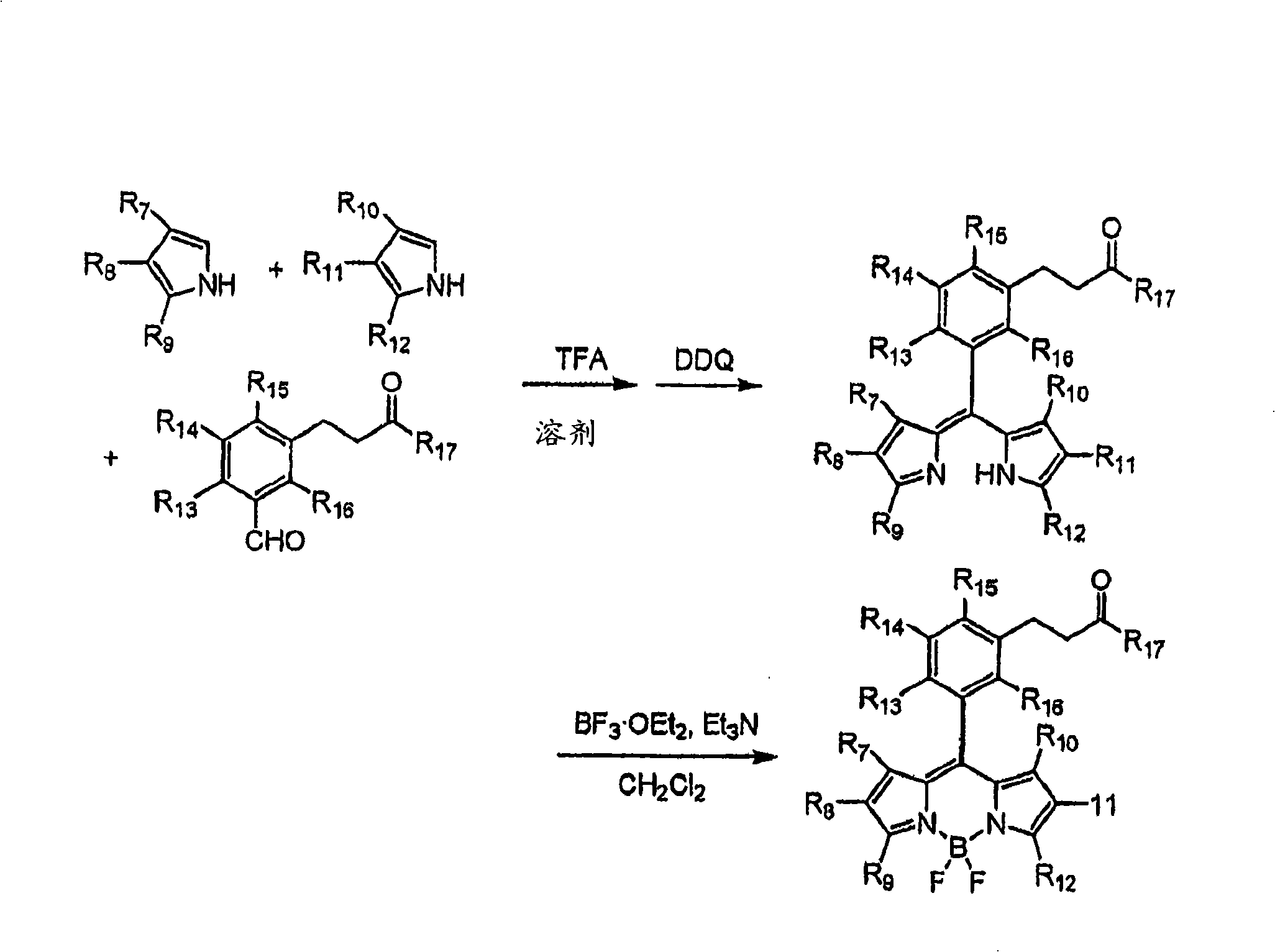
Embodiment 1
[0111] The synthetic scheme of embodiment 1-ss-6
[0112] 1) Synthesis of pyrrole-2-carboxylic acid (such as Figure 4 shown)
[0113] Pyrrole-2-carbaldehyde (10.0 g, 105 mmol) was dissolved in 50 mL of methanol, then diluted with 500 mL of distilled water. Freshly prepared silver oxide (48.3 g, 210 mmol) and sodium hydroxide (8.5 g, 212 mmol) were added. The reaction mixture was then stirred at room temperature for 1 hour. The precipitate was filtered off and washed with hot water. The combined filtrate and washings were extracted with ether (500 mL), then acidified with 37% hydrochloric acid at 0°C. The resulting solution was extracted with ether (200 mL x 4). The combined organic extracts were dried over magnesium sulfate. The solvent was evaporated under reduced pressure to obtain pyrrole-2-carboxylic acid [634-97-9] (9.9 g, yield 85%).
[0114] 2) Synthesis of N, N-diethyl-1H-pyrrole-2-carboxamide (ss-1) (such as Figure 4 shown)
[0115] Pyrrole-2-carboxylic aci...
Embodiment 2
[0131] 1) Fluorescence spectrum of ss-6
[0132] The compound ss-6 obtained in Example 1 was dissolved in CH 3 CN to a concentration of 2 mM, and then 100 mM sodium phosphate buffer (pH 7.4) was added to the solution to dissolve it to a final concentration of 20 μM. The excitation and fluorescence spectra of 20 μM ss-6 solution were measured with a PerkinElmer LS50 fluorescence spectrometer. The width of the slit used for measuring the excitation spectrum and the fluorescence spectrum is both 5 nanometers, and the voltage of the photomultiplier tube is 775 volts. Measurements were performed at an excitation light wavelength of 515 nm. The results obtained are shown in Figure 8 .
[0133] In order to study the relationship between ss-6 and peroxynitrite (ONOO - ), according to the method of Keith and Powell (Keith, W.G. & Powell, R.E.; Kinetics of decomposition of peroxynitrous acid; J.Chem.Soc.A., 1969,1,90) to prepare ONOO - solution. Briefly, a mixture of sodium nitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com