Coumarin fluorescent probe and preparation method and application thereof
A fluorescent probe and coumarin-based technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as damaging biomolecules, affecting cell processes, and apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of above-mentioned coumarin fluorescent probe is as follows:
[0032] S1. Dissolve 7-hydroxycoumarin and hexamethylenetetramine in acetic acid solvent and react at a temperature of 70-100°C. After the reaction, cool down to below 70°C and add hydrochloric acid to adjust the pH value of the system to 2-5. , extracted with ethyl acetate, and recrystallized from ethanol to obtain the formula (I ’ ) the first intermediate product of the structure shown;
[0033]
[0034] S2. Dissolve the first intermediate product, 2-aminothiophenol and sodium metabisulfite in N,N-dimethylformamide solvent, react at a temperature of 110-120°C, add water to precipitate a solid after the reaction, and filter to obtain The second intermediate product of the structure shown in formula (II);
[0035]
[0036] S3. Dissolving the second intermediate product, pinacol 4-bromomethylbenzene borate and potassium carbonate in acetonitrile solvent, reacting at 70-90°C, conc...
Embodiment 1
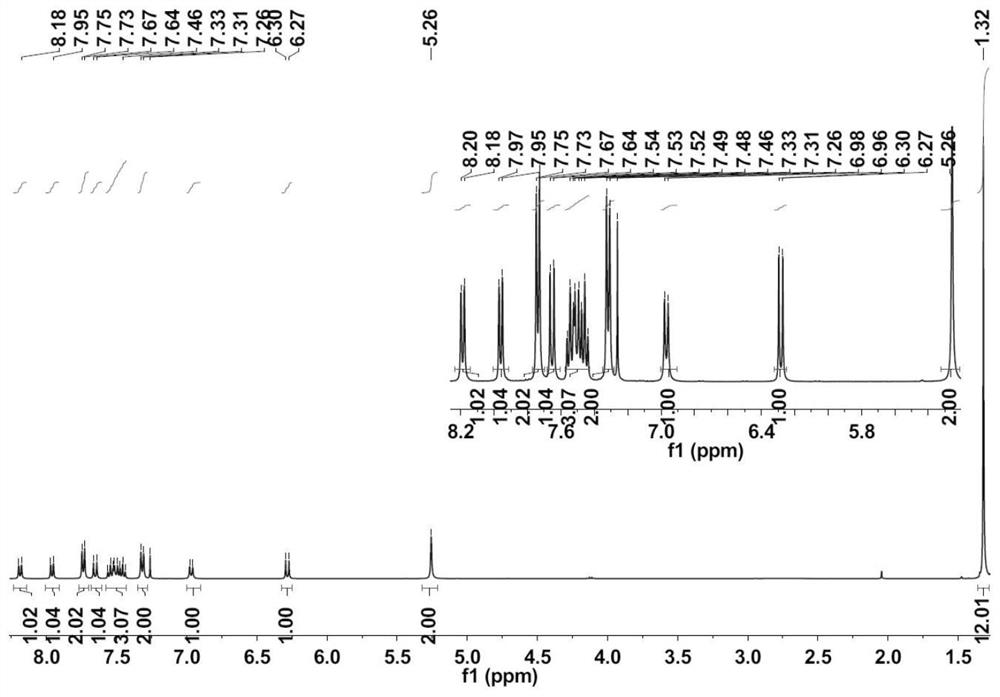
[0055] Embodiment 1: Preparation of coumarin fluorescent probe L
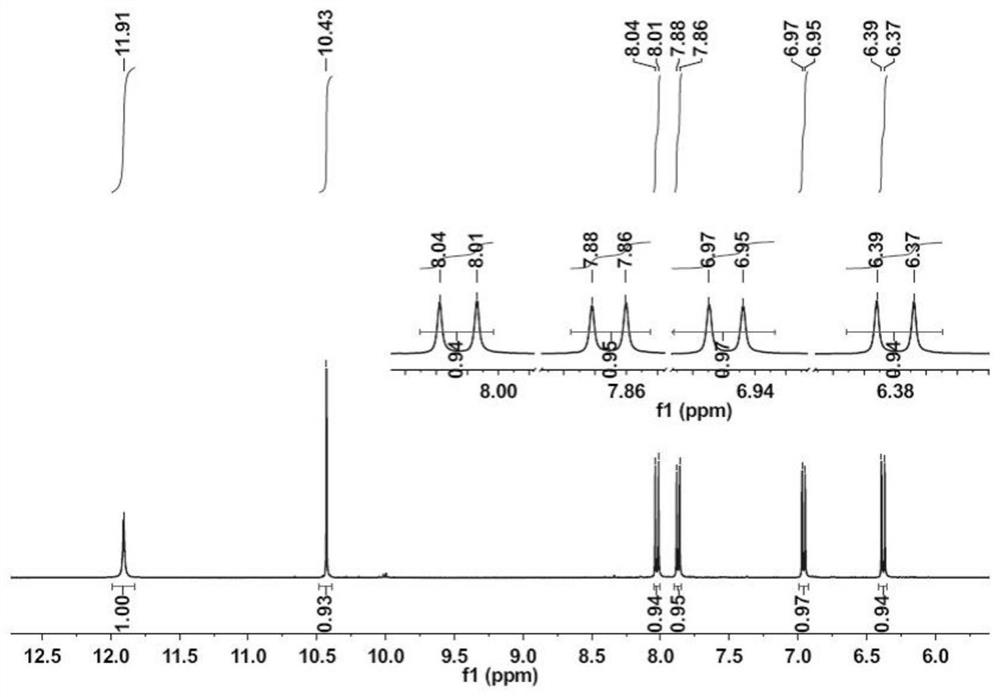
[0056] Synthesis of the first intermediate product: put 10g (61.6mmol) of 7-hydroxycoumarin (compound 1) and 4.8g (33.88mmol) of hexamethylenetetramine into a 100mL round bottom flask, add 30mL acetic acid Dissolve, heat to 90°C, stir and react for 8 hours to stop the reaction; cool down to 70°C, add 50mL of 1mol / L hydrochloric acid solution and stir to adjust the pH value to 2-5; add ice water, add ethyl acetate solvent extraction, extract Add 30 mL each time for three times, then dry the organic phase with anhydrous sodium sulfate, filter under reduced pressure, remove the solvent ethyl acetate by rotary evaporation of the filtrate under reduced pressure, and obtain a yellow solid; then recrystallize the yellow solid with absolute ethanol to obtain 1.8 g of the second An intermediate product, yield 18%.
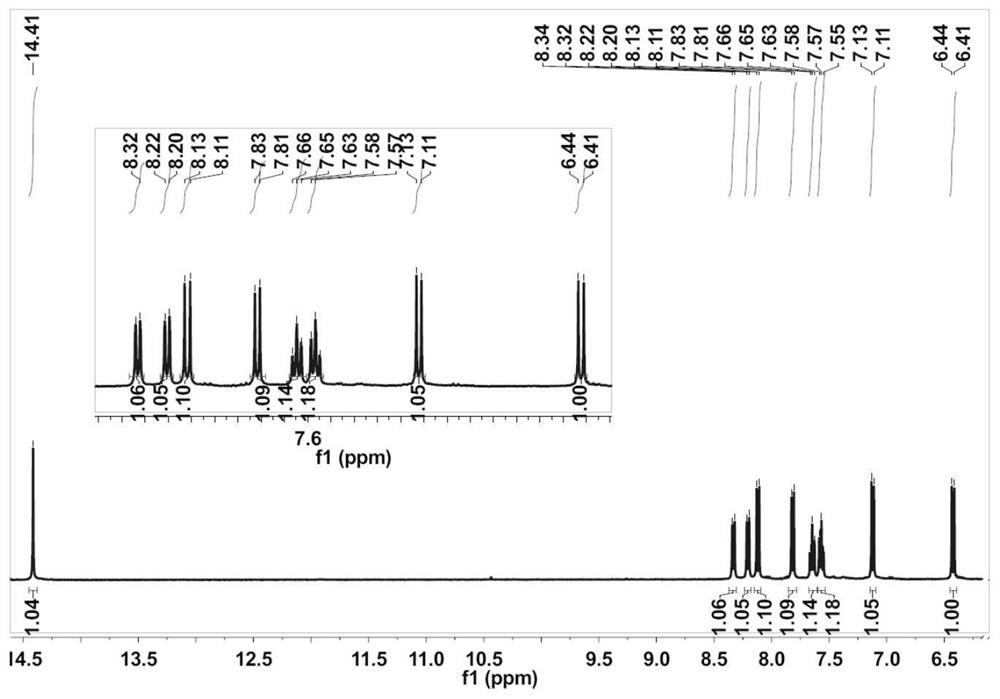
[0057] Synthesis of the second intermediate product: Put 0.69 g (3.67 mmol) of 7-hydroxycoumarin aldehyd...
Embodiment 2
[0060] Embodiment 2: coumarin fluorescent probe L to ONOO - selective detection of
[0061] Configure a coumarin-based fluorescent probe dimethyl sulfoxide standard solution with a molar concentration of 1.0 mmol / L;
[0062] In a mixed buffer solvent of DMSO and PBS (v:v=80:20), add the coumarin fluorescent probe dimethyl sulfoxide standard solution and the analyte solution with a molar concentration of 10mmol / L and 0.7 mmol / L ONOO - solution; after stirring evenly, wait for 15 minutes to detect the change of the fluorescence emission spectrum of the solution;
[0063] Among them, the analytes include: Al 3+ , Ca 2+ 、Cu 2+ , Fe 2+ , Fe 3+ , Hg 2+ , Mg 2+ , Pb 2+ , Zn 2+ , Cys, GSH, Hcy, HNO 3 , NO, t Bnoo · 、H 2 o 2 , OH, ClO - ;
[0064] Such as Figure 4 As shown, when not adding any analyte, the fluorescent probe L solution has almost no emission peak at 500nm, when adding ONOO - Finally, the fluorescent probe solution showed a strong emission peak at 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com