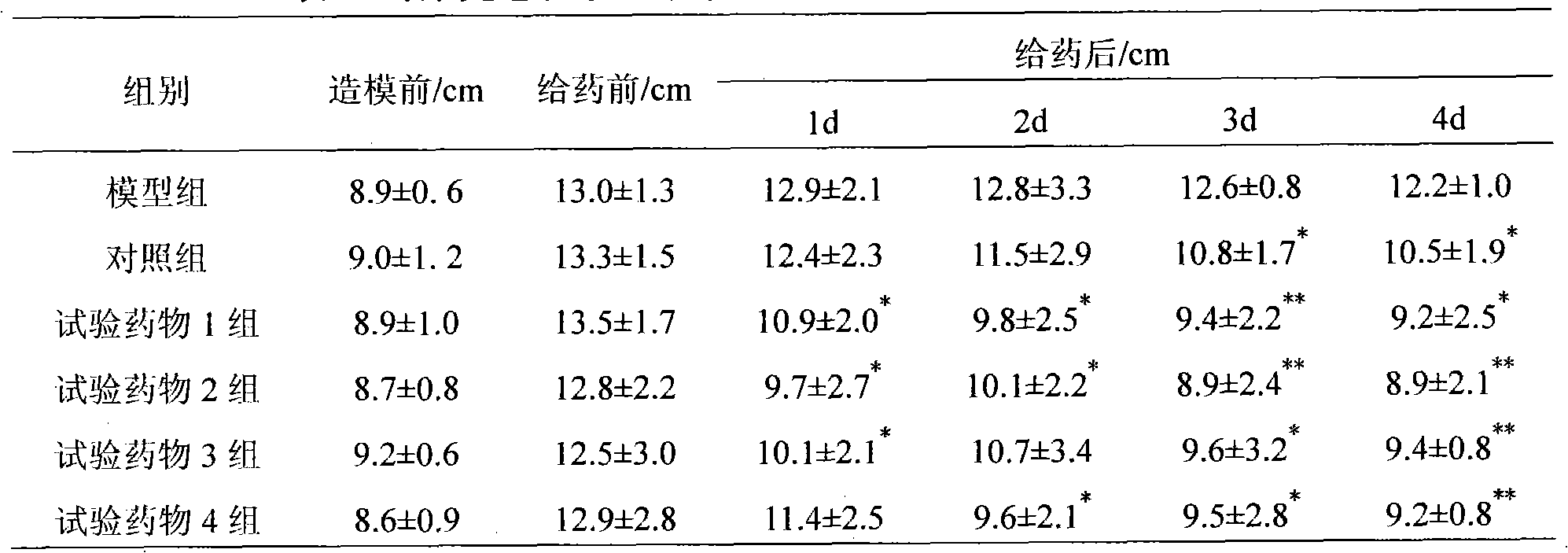
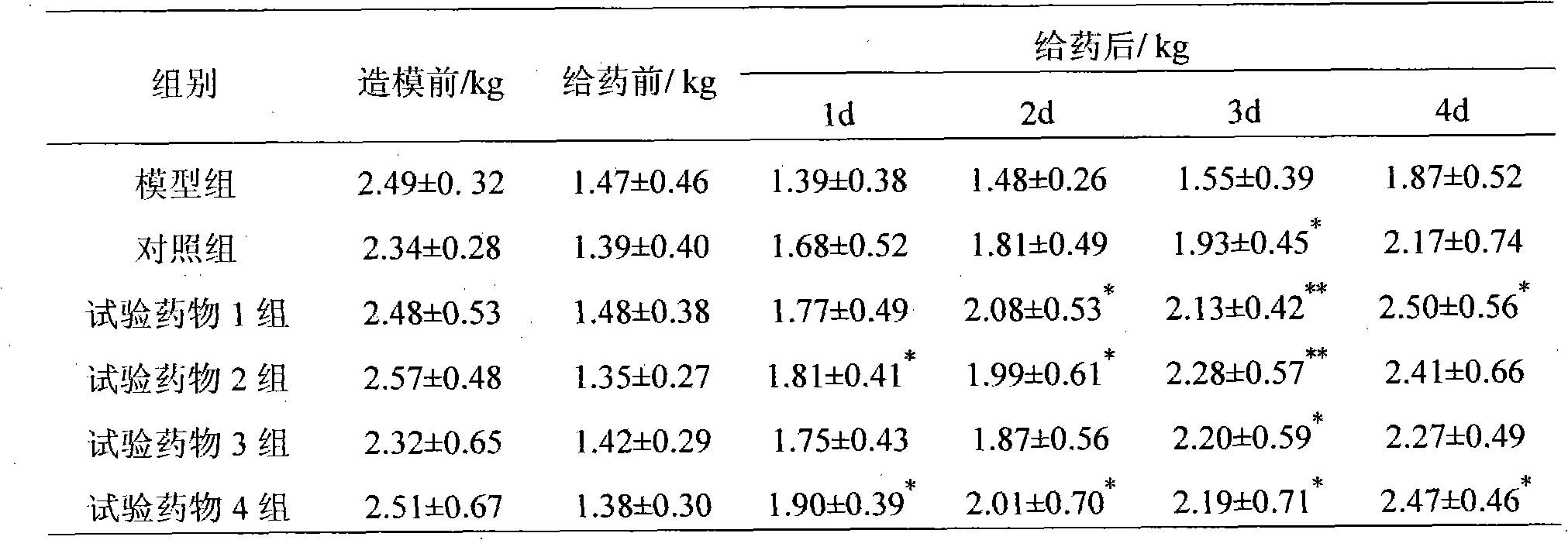
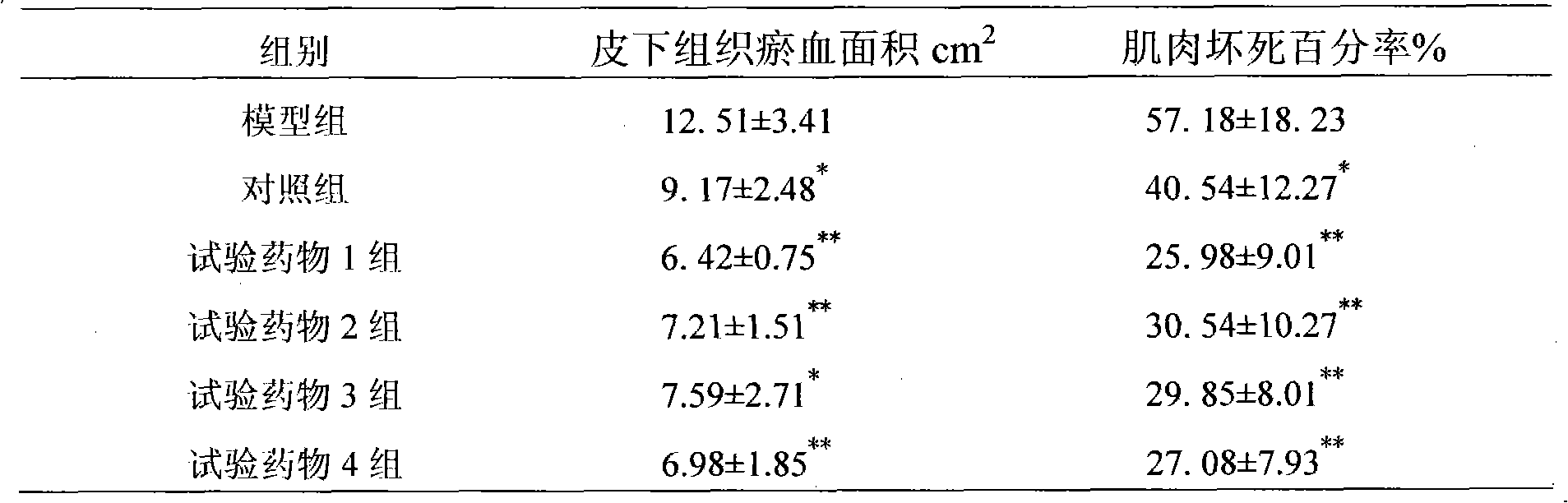
Method for preparing Liaoyuanqili super fine powder
A technology of ultra-fine powder and Qilisan, which is applied in the field of Liaoyuan Qilisan ultra-fine powder preparation, can solve the problems of high cost of liquid nitrogen freezing and crushing technology, difficulty in achieving ultra-fine crushing, and limited development and utilization. It is beneficial to quality control, prolonging the shelf life and reducing the consumption of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] This embodiment prepares Liaoyuan Qilisan ultrafine powder by a method comprising the following steps:
[0017] (1) Drying: Except that frankincense, myrrh and borneol do not need to be dried, other raw materials (including dried blood, safflower, catechu, angelica, ground beetle, Sanqi, drynaria, rhubarb, Fanghai, Fragrance) dried in a drying oven for 8 hours at a drying temperature of about 50°C;
[0018] (2) Coarse crushing: prepare each raw material according to the proportion of the drug formula (50g of dried blood, 450g of safflower, 16g of borneol, 240g of frankincense, 200g of myrrh, 160g of catechu, 90g of angelica, 80g of ground beetle, 50g of Panax notoginseng, broken bone Make up 240g, rhubarb 240g, fanghai 240g, jiangxiang 240g), dry blood is mixed with frankincense and myrrh, and adopt impact crusher to carry out coarse pulverization, until the granularity is 50~60 mesh mixed coarse powder I; Type crusher, the other raw medicinal materials in Liaoyuan Qil...
Embodiment 2
[0021] This embodiment prepares Liaoyuan Qilisan ultrafine powder by a method comprising the following steps:
[0022] (1) Drying: Except that frankincense, myrrh and borneol do not need to be dried, other raw materials (including dried blood, safflower, catechu, angelica, ground beetle, Sanqi, drynaria, rhubarb, Fanghai, Fragrance) dried in a drying oven for 4 hours at a drying temperature of about 65°C;
[0023] (2) Coarse crushing: prepare each raw material according to the proportion of the drug formula (50g of dried blood, 450g of safflower, 16g of borneol, 240g of frankincense, 200g of myrrh, 160g of catechu, 90g of angelica, 80g of ground beetle, 50g of Panax notoginseng, broken bone Make up 240g, rhubarb 240g, Fanghai 240g, Jiangxiang 240g), dry blood, safflower, frankincense, myrrh are mixed, adopt shearing crusher to carry out coarse pulverization, to granularity is the mixed coarse powder 1 of 70~80 purpose; Then, the shearing crusher is also used to coarsely pulve...
Embodiment 3
[0026] This embodiment prepares Liaoyuan Qilisan ultrafine powder by a method comprising the following steps:
[0027] (1) Drying: Except that frankincense, myrrh and borneol do not need to be dried, other raw materials (including dried blood, safflower, catechu, angelica, ground beetle, Sanqi, drynaria, rhubarb, Fanghai, Fragrance) dried in a drying oven for 10 hours at a drying temperature of about 30°C;
[0028] (2) Coarse crushing: prepare each raw material according to the proportion of the drug formula (50g of dried blood, 450g of safflower, 16g of borneol, 240g of frankincense, 200g of myrrh, 160g of catechu, 90g of angelica, 80g of ground beetle, 50g of Panax notoginseng, broken bone Make up 240g, rhubarb 240g, Fanghai 240g, Jiangxiang 240g), safflower, notoginseng are mixed with frankincense, myrrh, adopt crystal breaker to carry out coarse pulverization, to granularity is 60~70 purpose mixed coarse powder I; Then similarly Using a crystal crusher, mix other raw medi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com