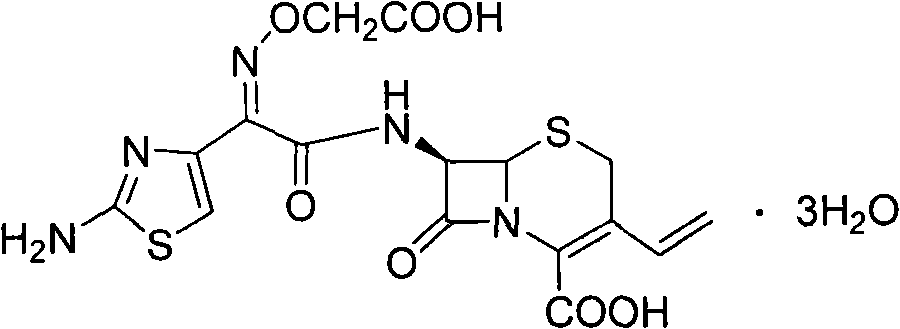
Synthetic method of antibiotic cefixime
A technology of cefixime and synthetic method, applied in the direction of antibacterial drugs, organic chemistry, etc., can solve problems such as unfavorable continuity, large-scale industrial production, expensive tetrahydrofuran, harsh reaction conditions, etc., to shorten the production cycle and simplify Effects on manipulation, yield enhancement and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
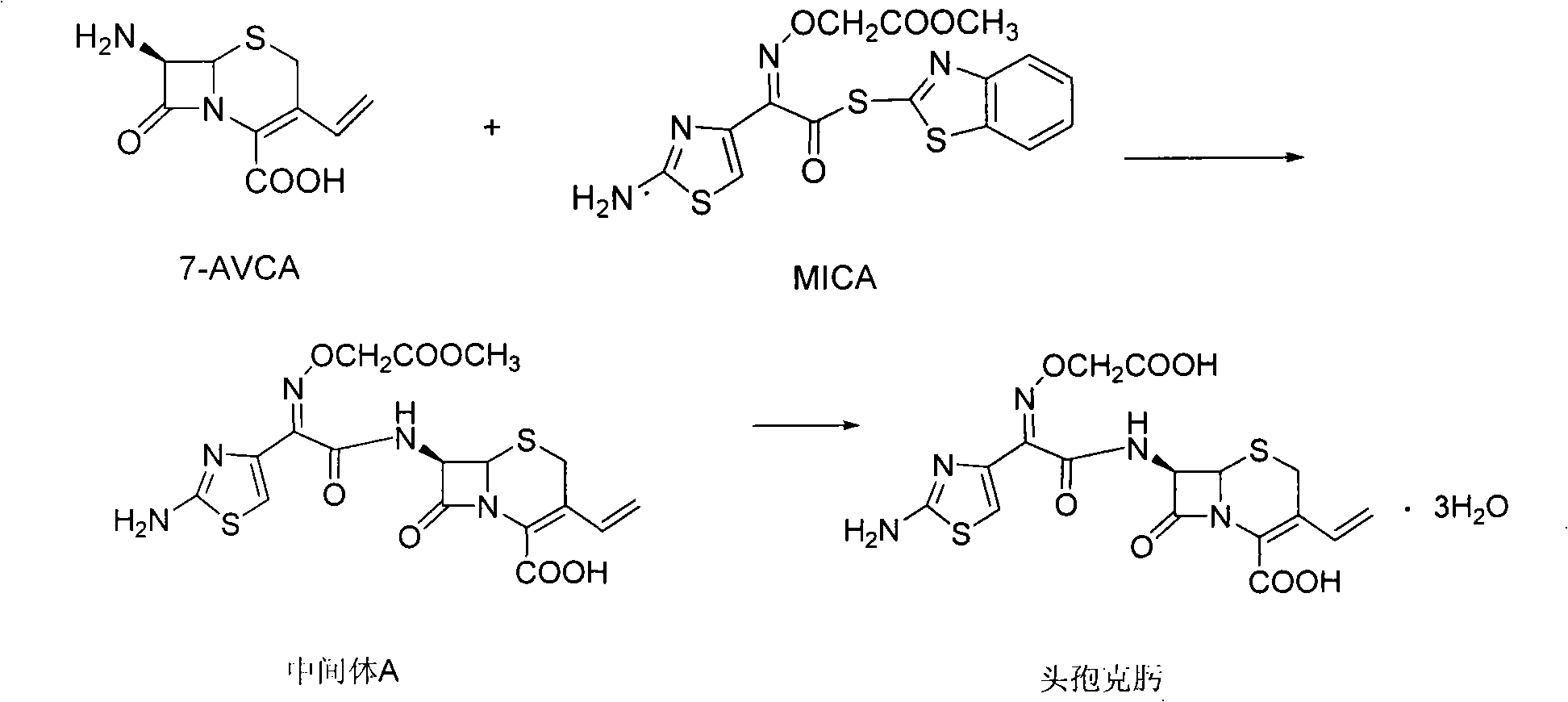
Embodiment 1
[0016] In a 1000L reactor, add 500L acetone, 50kgAVCA, 75kgMICA-active ester, 50L methanol, add 25kg triethylamine dropwise at 5°C for about 1.5h (hour), and keep the reaction at 5°C for 4h (hour), Sampling (intermediate less than 0.3%).
[0017] After the reaction is over, acetone and methanol are recovered by distillation under reduced pressure at 40°C. The recovered acetone and methanol can be used directly. After steaming, add 400L of pure water and 400L of ethyl acetate to cool down to 10°C. The water phase was extracted twice by adding 200L ethyl acetate to the water phase, and the water phase was collected and filtered to remove insoluble matter.
[0018] Add 8 kg of activated carbon to the water phase, stir for 30 min, filter, then wash the filter cake with 200 L of pure water, and combine the filtrate and washing liquid.
[0019] Add 200L of acetone to the water phase and lower the temperature to below -10°C, add 80L of 30% sodium hydroxide at one time, stir for 5min...
Embodiment 2
[0022] In a 1000L reactor, add 500L acetone, 50kgAVCA, 90kgMICA-active ester, 50L pure water, add 25kg triethylamine dropwise at 10 degrees for about 2.5 hours, keep warm at 5 degrees for 4 hours, and take samples (intermediate less than 0.3 %).
[0023] After the reaction is over, acetone is recovered by distillation under reduced pressure at 45°C. The recovered ketone can be used directly. After steaming, add 500L of pure water and 400L of ethyl acetate to cool down to 10°C, keep the layers for 30 minutes, collect the water phase, water The phase was extracted twice by adding 200L ethyl acetate, and the aqueous phase was collected and filtered to remove insoluble matter.
[0024] Add 3 kg of activated carbon to the water phase, stir for 30 min, filter, then wash the filter cake with 200 L of pure water, and combine the filtrate and washing liquid.
[0025] Add 200L of acetone to the water phase and lower the temperature to below -5°C, add 100L of 20% sodium hydroxide at one...
Embodiment 3
[0028] The weight ratio of 7-AVCA and MICA active ester is that 1: 1.5 is mixed and placed in the mixed solvent that the volume ratio of acetone and methanol is 1: 30 and the volume ratio of pure water water is 1: 30, with sodium hydroxide or Potassium hydroxide adjusts the pH value to 7.0, dissolves and undergoes an acylation reaction at a temperature of -20°C to obtain a cefixime intermediate A solution; the obtained cefixime intermediate A solution is extracted with ethyl acetate solvent, and after extraction In the water phase obtained, add 0.1% activated carbon of 7-AVCA charging capacity in water phase and carry out decolouring, then add 0.1% EDTA of 7-AVCA charging capacity, obtain cefixime intermediate A aqueous solution; The weight ratio is 1:1,
[0029] When the acylation reaction is over, acetone and methanol are recovered by distillation under reduced pressure at 40°C. The recovered acetone and methanol can be used directly. After steaming, add 3 times the amount o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com