Method for preparing nano-polymers of hydroxyl sulfoacid phenylamine and pyrrole
A nano-technology of hydroxysulfonic acid aniline and pyrrole, which is applied in the direction of organic material conductors and non-metallic conductors, can solve problems such as environmental pollution and unsatisfactory nano-PPy effects, and achieve simple post-treatment process, excellent hydrophilicity, and stability sex good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
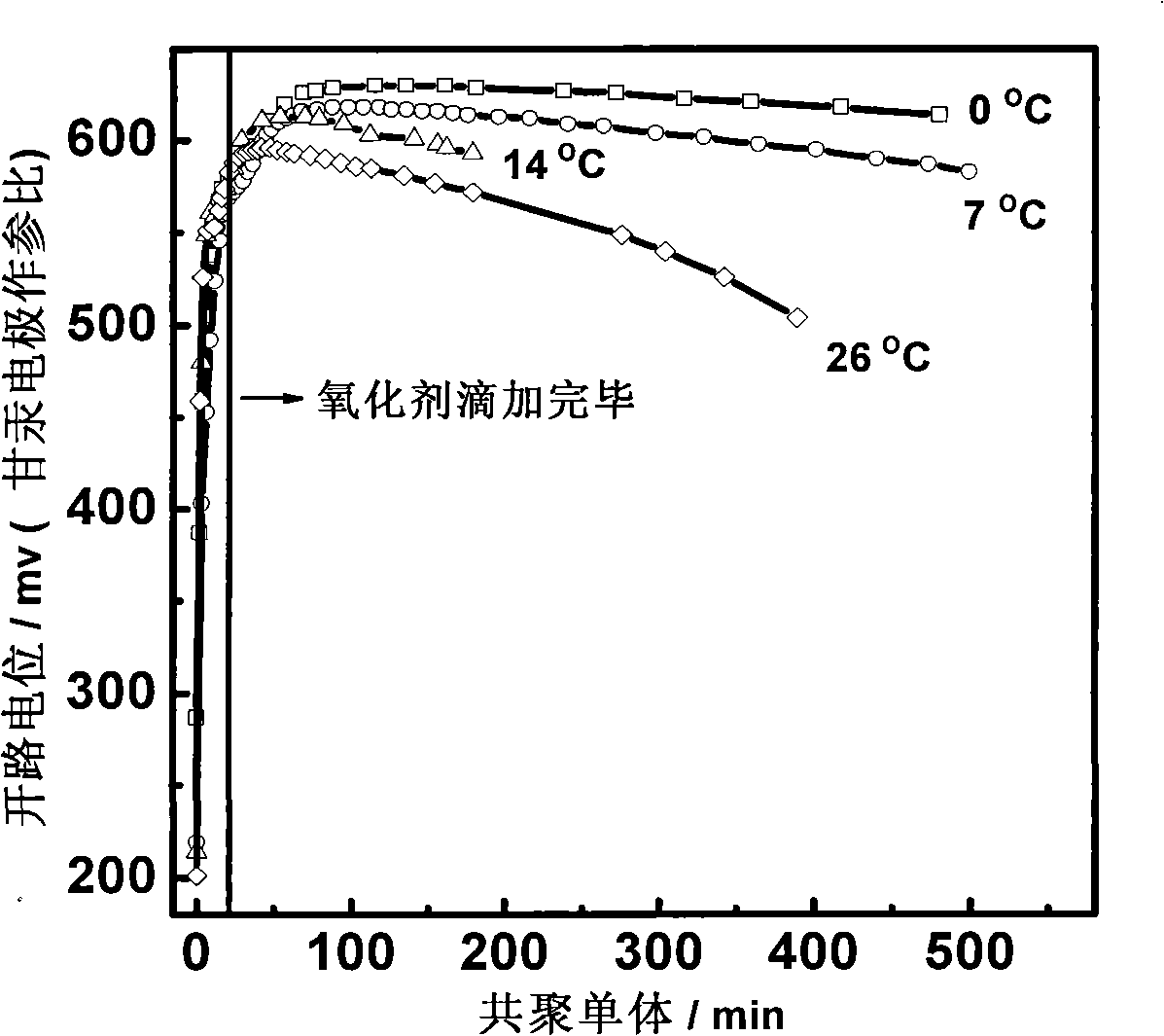
[0038] Accurately weigh 0.946g (5mmol) hydroxysulfonate aniline (HSS) monomer into a beaker filled with 100mL 1mol / L HCl aqueous solution, stir until completely dissolved, and the solution is light pink at this time. Use a pipette to measure 0.35mL (5mmol) pyrrole (Py) monomer into this solution, stir until the pyrrole is completely dissolved, and then filter to remove a small amount of insoluble impurities to obtain a mixed solution of the two monomers. At this time, the solution is transparent. brownish yellow. Weigh 2.282g (10mmol) of ammonium persulfate and dissolve it in 20mL of 1mol / L HCl aqueous solution to make an oxidizing agent solution for later use. Put the prepared mixed monomer solution and oxidant solution in a water bath at 14°C to keep the temperature for 30 minutes, then drop the oxidant into the monomer solution, and control the dropping rate to 1 drop / second, and it takes about 20 minutes to complete the oxidant dropwise. The solution color gradually chang...
Embodiment 2-28
[0042] Repeat Example 1, the total number of moles of monomers is constant, and the molar ratio and reaction temperature of comonomers are changed. See Table 1 for specific data.
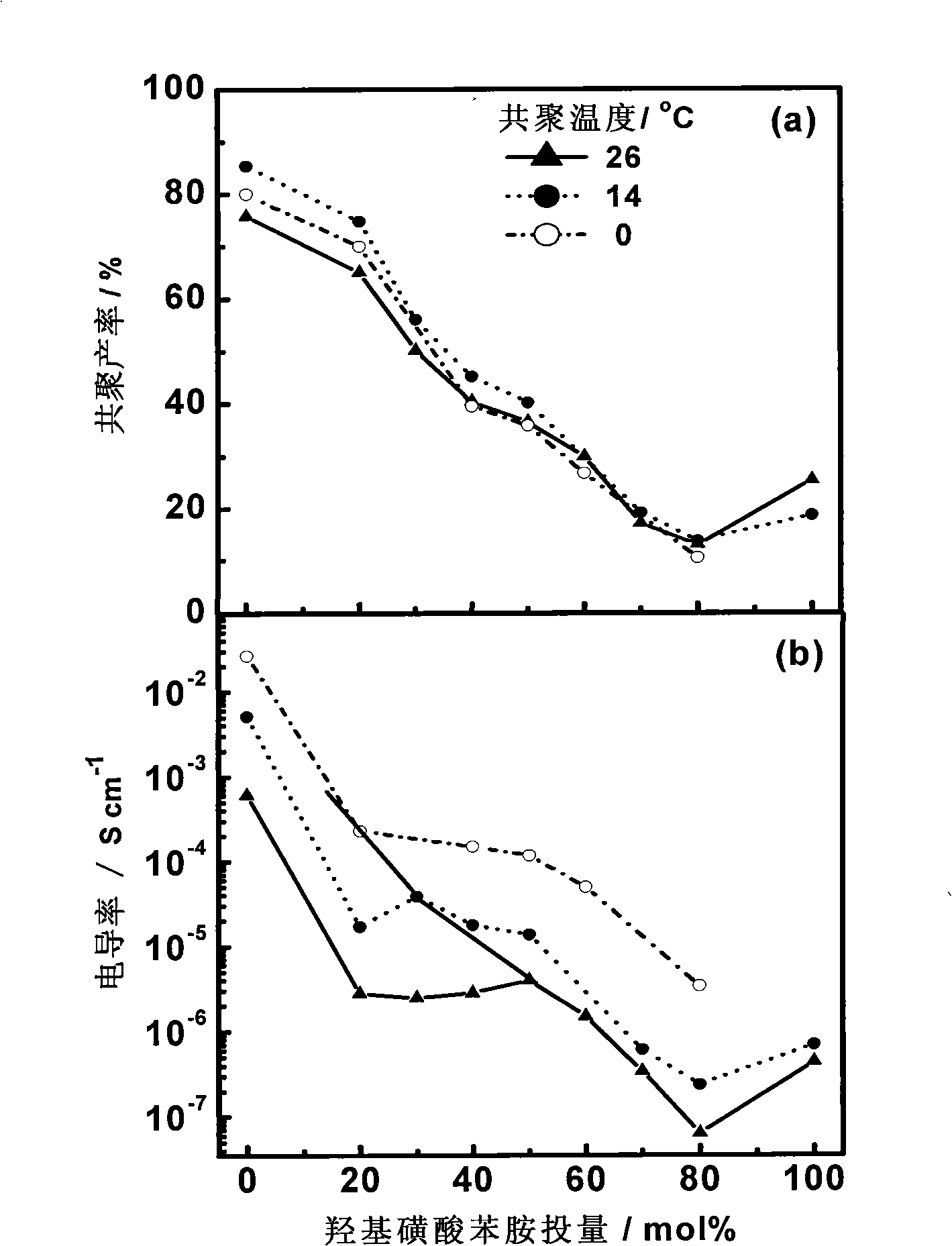
[0043] figure 1 In 1mol / L HCl at three polymerization temperatures, the yield and conductivity of the copolymerized product obtained by polymerization for 24 hours under the condition that the oxygen / mono ratio is 1 varies with the comonomer ratio. It can be seen that in the three At the polymerization temperature, with the increase of the input amount of hydroxysulfonic acid aniline monomer, the yield of copolymer decreased linearly, which was related to the low polymerization activity of hydroxysulfonic acid aniline in the copolymerization system. When the input amount of hydroxysulfonic acid aniline monomer reaches 80mol%, the copolymer yield has a minimum value, indicating that the copolymerization activity of the two monomers is low at this time, which is not conducive to the occurrence of cop...
Embodiment 29-30
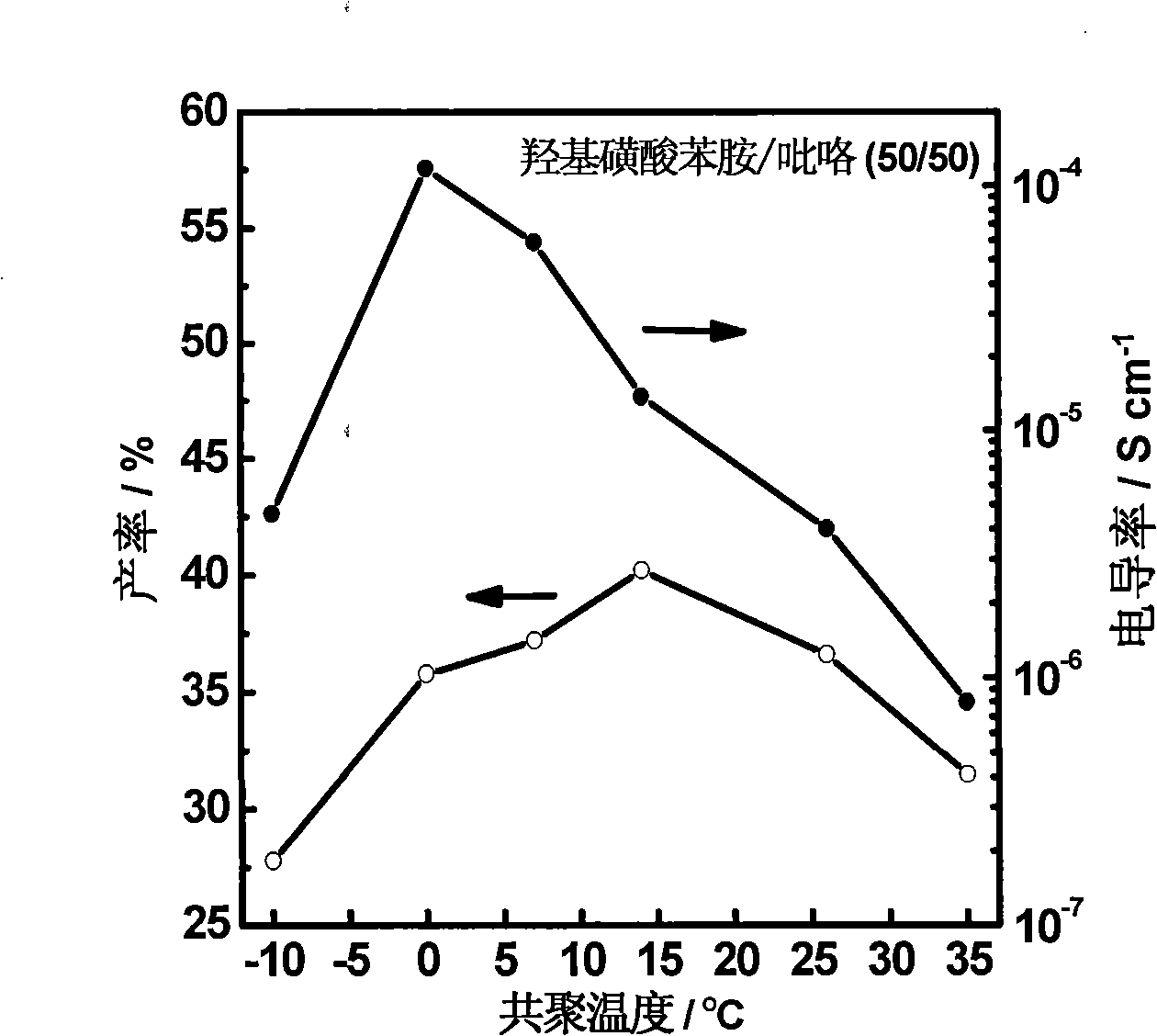
[0062] Repeat Example 1, the total number of moles of monomers remains unchanged, the molar ratio of comonomers is 1:1, and the reaction temperatures are -10°C, 7°C, and 35°C. Changes in the polymerization yield and electrical conductivity of the copolymerization system with a monomer molar ratio of 50 / 50 in the range of -10°C to 35°C. see results image 3 , and the corresponding data are listed in Table 4. The copolymerization reaction is exothermic, so lowering the temperature can make the reaction move toward the direction of copolymerization, that is to say, low temperature is beneficial to the copolymerization reaction. When the temperature is lower, the reaction of the system is slower, and the polymerization is in a more controllable state, which leads to the increase of α-α linkage between pyrrole monomers, the increase of the plane conjugation of the molecular chain of the whole copolymer, and the higher conductivity. However, if the polymerization temperature is to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com