Preparation method of glimepiride tablet
A technology for glimepiride and urea tablets, which is applied in the field of preparation of glimepiride tablets, can solve the problems that the dissolution rate is not easy to reach the quality standard, affects the production and marketing of glimepiride tablets, and cannot improve the properties of the dissolution rate, etc. Solve the effects of hydrophobicity and insolubility, easy decomposition at high temperature, increase disintegration and dissolution rate, and improve quality stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 exceptionally beautiful urea tablet
[0034] (1) The raw material of glimepiride and micropowder silica gel are co-ground by micronization method, and passed through a 100 mesh sieve to obtain the mixed powder of micronized glimepiride and micropowder silica gel, wherein the weight ratio of glimepiride to micropowder silica gel 1:1;
[0035] (2) The mixed powder prepared in step (1), mannitol, calcium hydrogen phosphate, pregelatinized starch and starch filler, povidone binder, sodium carboxymethyl starch disintegrant, magnesium stearate lubrication Mix the ethanol solution with a volume concentration of 35% at room temperature for 15 minutes, pass through a 20-mesh sieve to granulate, dry the granules at 60°C, and then sieve the 20-mesh sieve for granulation. Check the content of the granules to determine the weight of the tablet. Meurea tablets.
[0036]Wherein, the amount of the mixed powder in the step (2): the glimepiride in the mi...
Embodiment 2
[0037] Example 2 Detection of Geweimeiurea Tablets
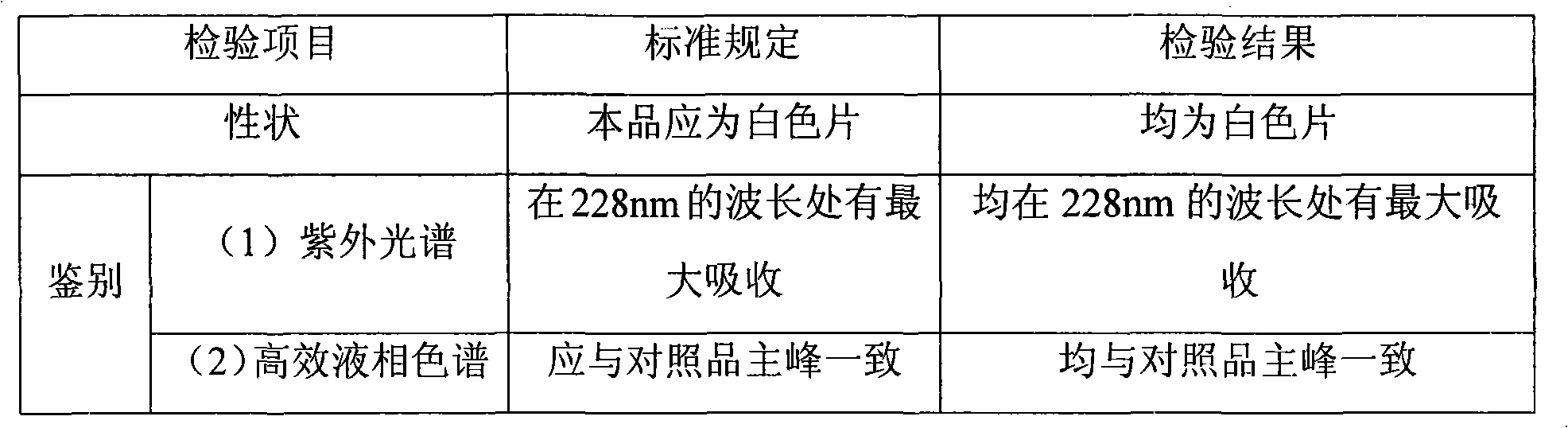
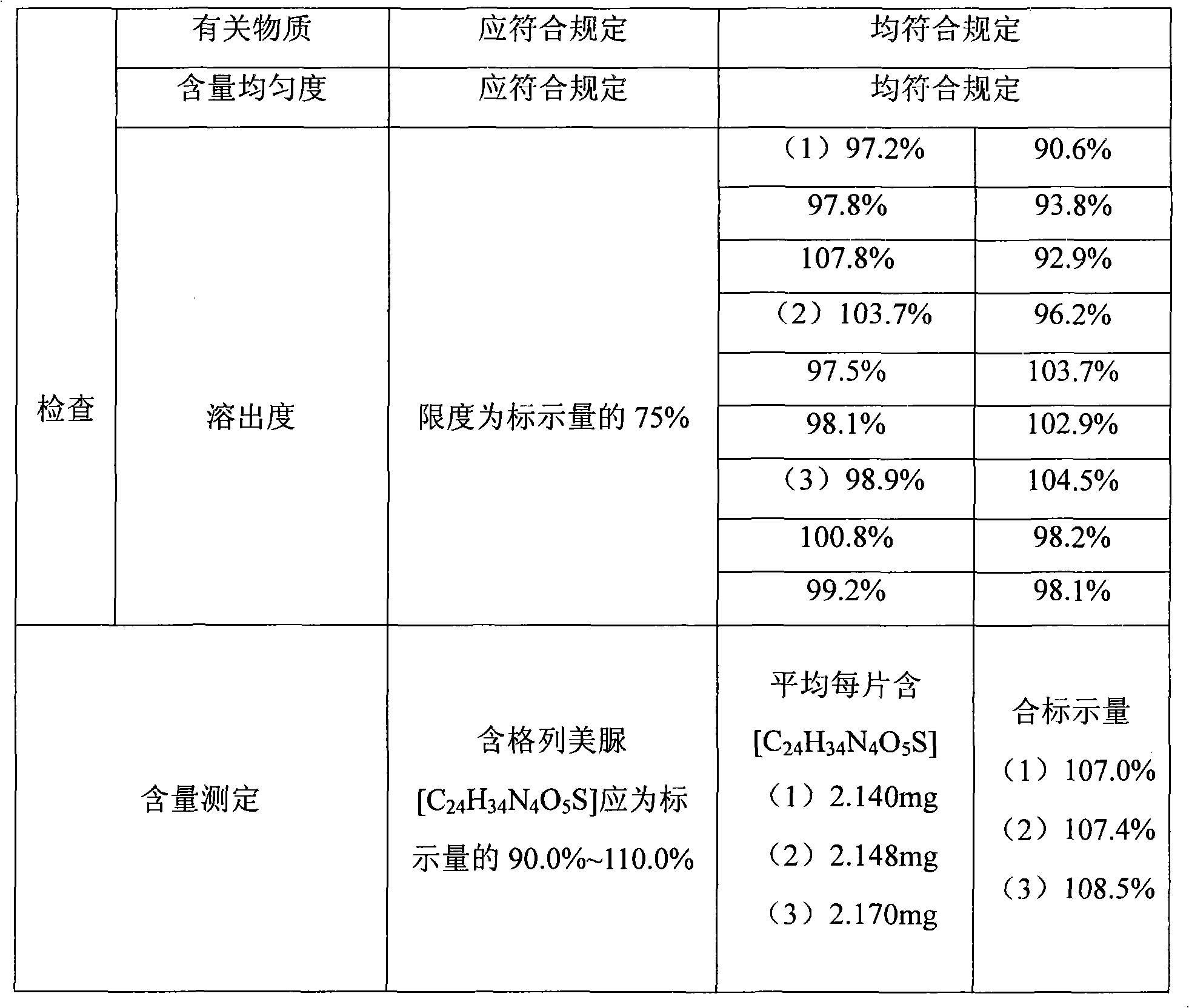
[0038] 1. Testing standard [National Drug Administration Standard (Trial) WS-102(X-087)-2001]
[0039] This product is especially meurea (C 24 h 34 N 4 o 5 S) should be 90.0% to 110.0% of the labeled amount.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com