21-deoxymacbecin analogues useful as antitumor agents
A technology of deoxymacbecin and macbecin, applied in the direction of antineoplastic drugs, resistance to vector-borne diseases, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
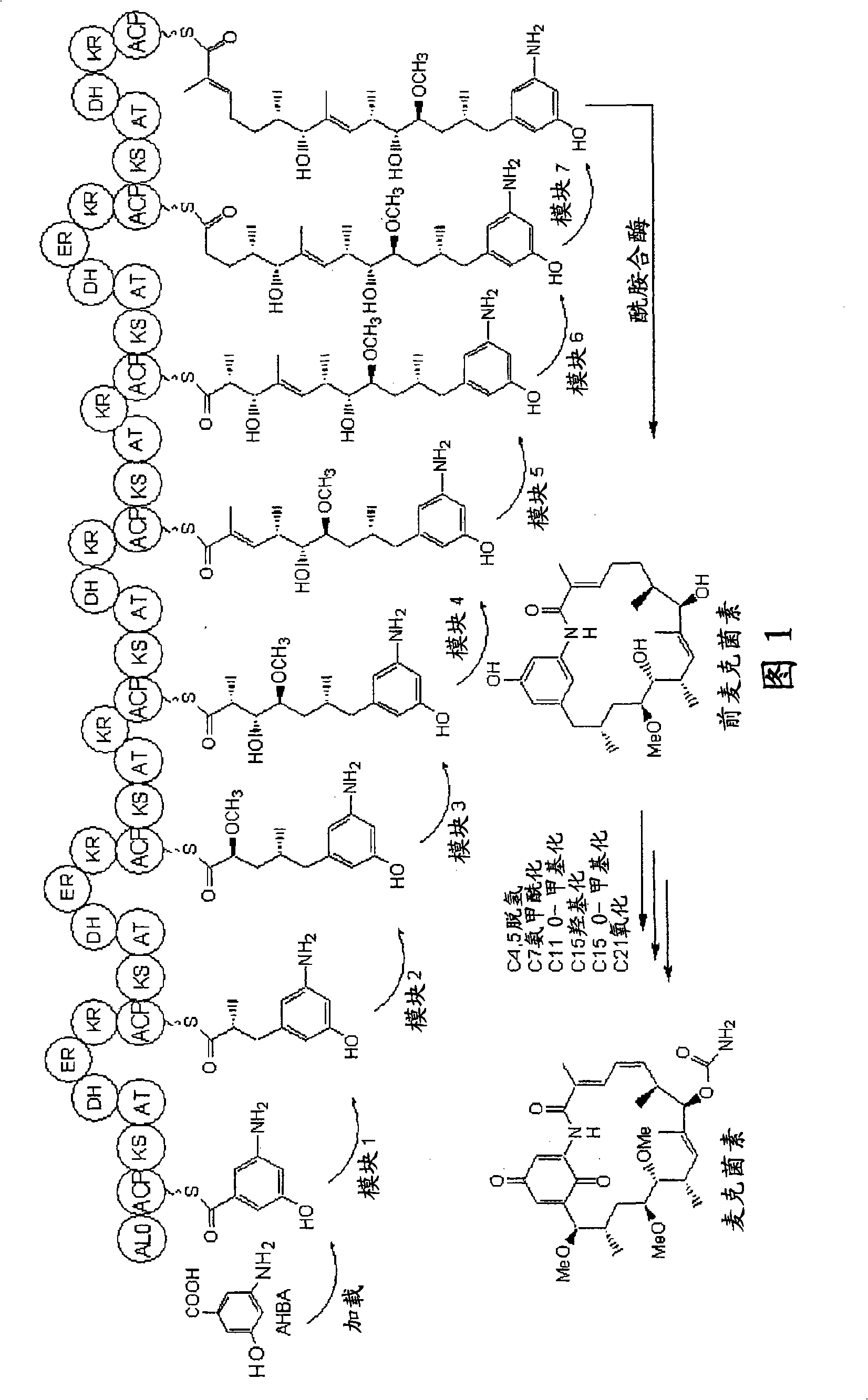
[0210] Example 1 - Sequencing of the macbecin biosynthetic gene cluster
[0211] Genomic DNA was isolated from A. amaranthus (ATCC 31280) and A. miraculous (DSM 43827, ATCC 29888) using the standard protocol described in Kieser et al. (2000). DNA sequencing was carried out using standard methods at the Sequencing Facility at the University of Cambridge's Department of Biochemistry, Tennis Court Road, Cambridge CB21QW.
[0212] Using primers BIOSG1045'-GGTCTAGAGGTCAGTGCCCCCGCGTACCGTCGT-3' (SEQ ID NO: 7) and BIOSG1055'-GGCATATGCTTGTGCTCGGGCTCAAC-3' (SEQ ID NO: 8), amplified from Streptomyces hygroscopicus NRRL 3602 (sequence accession number: AY179507) using standard techniques The carbamoyltransferase-encoding gene gdmN in the geldanamycin biosynthesis gene cluster. Southern blot experiments were performed according to the manufacturer's instructions (Roche) using DIG reagent and a kit for non-radioactive nucleic acid labeling and detection. DIG-labeled gdmN DNA fragments wer...
Embodiment 2
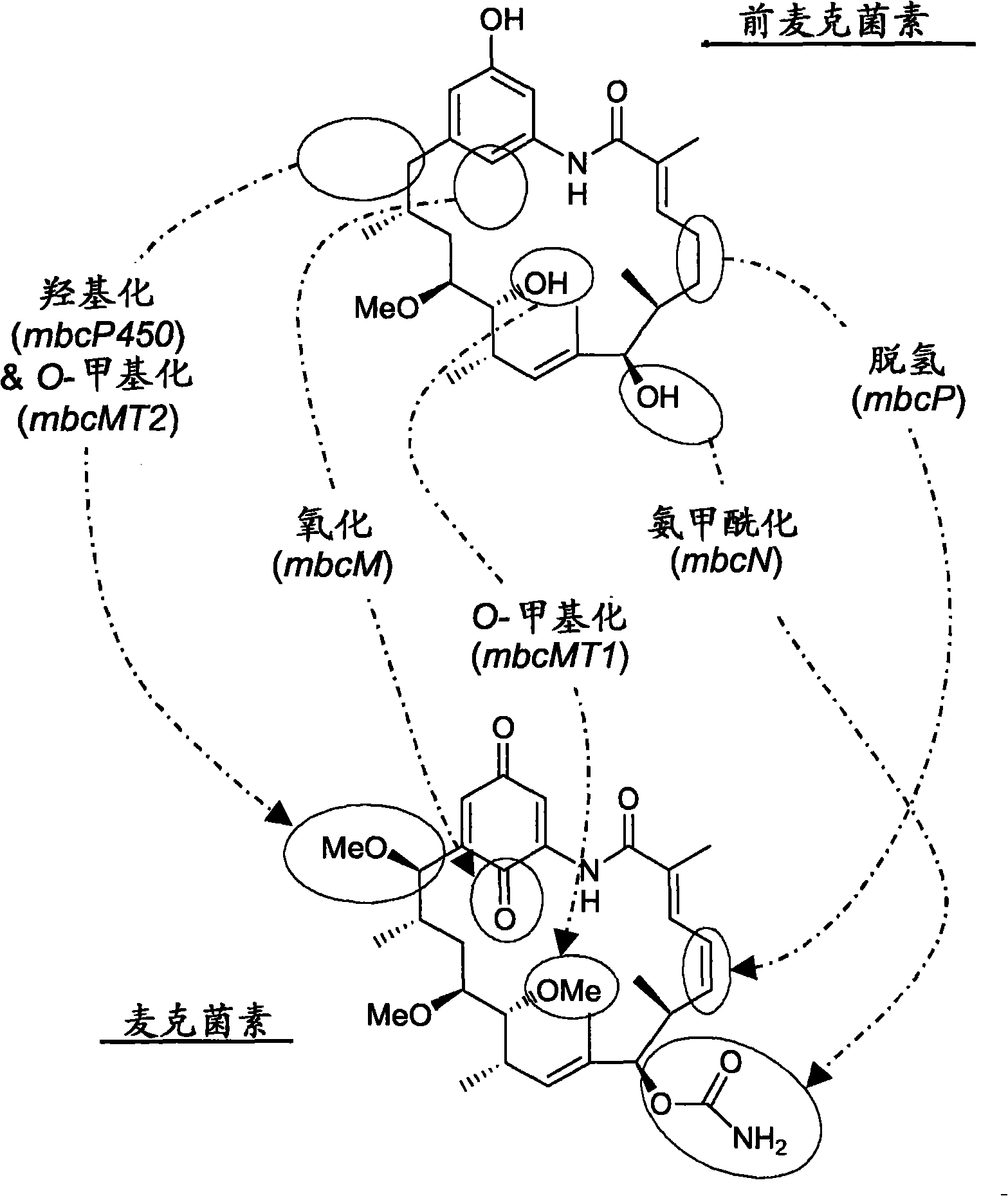
[0220] Example 2 - Production of Strain BIOT-3806: An Actinomyces acarina in which the gdmM homologue mcbM has been disrupted by inserting a plasmid
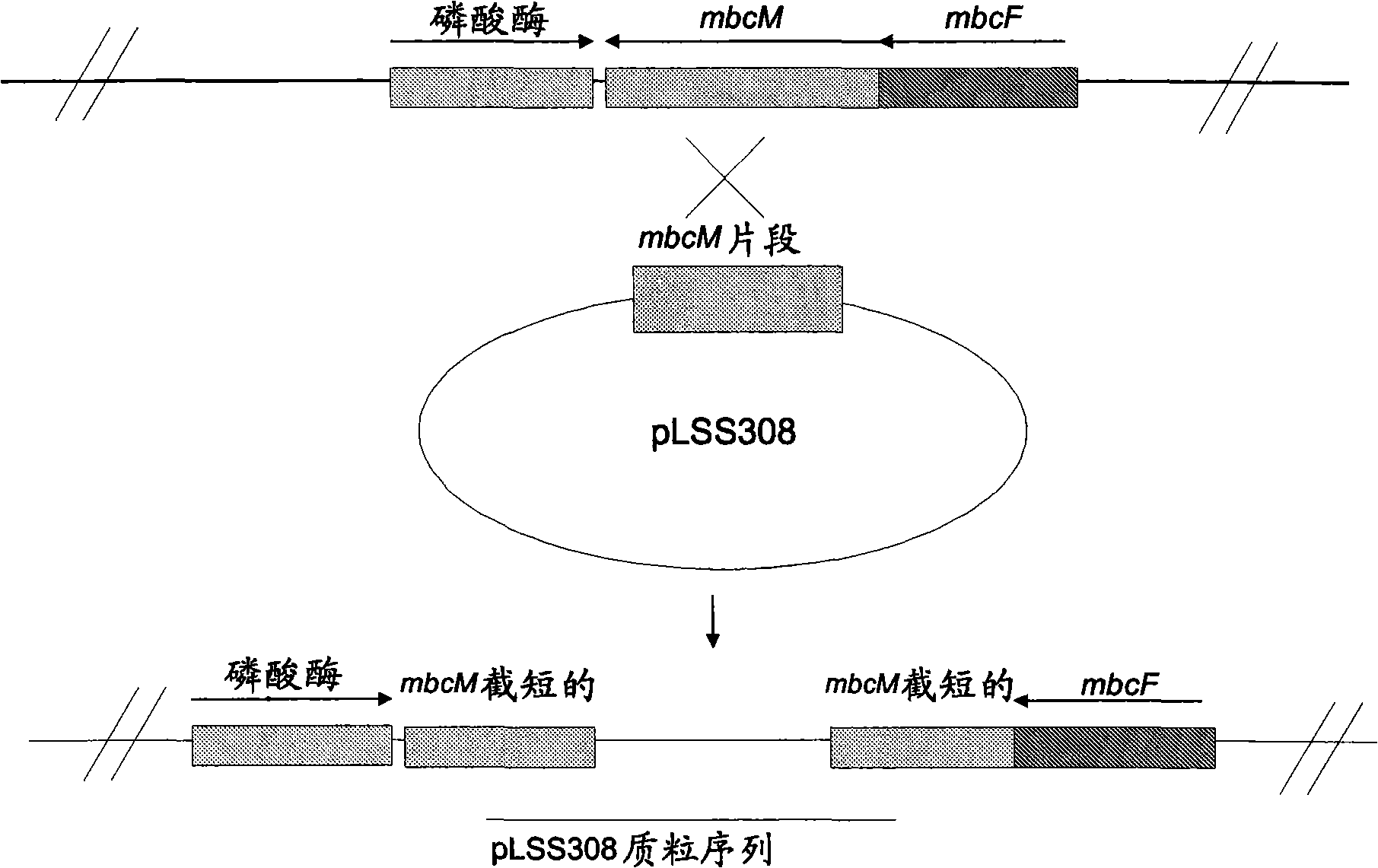
[0221] A summary of the construction of pLSS308 is at image 3 displayed in .
[0222] 2.1. Construction of plasmid pLSS308
[0223] DNA sequences of the gdmM gene from the geldanamycin biosynthesis gene cluster of Streptomyces hygroscopicus strain NRRL 3602 (AY179507) and orf19 from the rifamycin biosynthesis gene cluster of Amycolatopsis mediterranei (AF040570AF040571) Sequences were aligned using the VectorNTI sequence alignment program (Figure 4). This alignment identifies regions of homology suitable for the design of degenerate oligomers used to amplify DNA from Actinomyces fasciculatus (BIOT-3134; DSM43827; ATCC29888). Fragments of homologous genes. The degenerate oligomers are:
[0224] FPLS1: 5': ccscgggcgnycngsttcgacngygag 3'; (SEQ ID NO: 18)
[0225] FPLS3: 5': cgtcncggannccggagcacatgccctg 3'; (SEQ ID NO: 19) ...
Embodiment 3
[0237] Example 3 - Generation and isolation of new compounds
[0238] 3.1 Fermentation and isolation of 7-O-carbamoyl promacbecin
[0239] Nutrient stock solutions of BIOT-3806 were prepared after growth in Medium 1 containing 50 mg / L ampramycin and stored in distilled water with 20% w / v glycerol: 10% w / v lactose and at -80°C storage. Nutrient stocks were recovered on ISP2 medium (medium 3) plates supplemented with 50 mg / L ampramycin and incubated at 28°C for 48 hours. Vegetative cultures were obtained by removing two 5 mm diameter agar pieces from the ISP2 plate and inoculating them into 30 mL of Medium 1 containing 50 mg / L ampramycin in a 250 mL shake flask. The flasks were incubated at 28°C for 48 hours at 200 rpm (5 cm stroke).
[0240] The vegetative culture was inoculated at 5% v / v into 200 mL of production medium (Medium 2) in 2L shake flasks. Culturing was performed at 300 rpm (2.5 cm stroke) at 28°C for 1 day followed by 5 days at 26°C.
[0241] The fermentatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com